Abstract
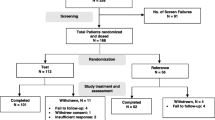
Rituximab (anti-CD20 monoclonal antibody) has shown to improve symptoms in rheumatoid arthritis (RA) patients with inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs). An anti-CD20 monoclonal antibody (Reditux™) developed by Dr. Reddy’s Laboratories, India, is currently approved for use both in rheumatology and oncology patients. This retrospective report evaluates the efficacy and safety data from the real-world use of Reditux™ over a 6-month period in Indian patients with RA. All consecutive moderate to severe RA patients who failed therapy with at least two DMARDs including methotrexate (MTX) for 6 months, TNFα inhibitor naive, and willing to take Reditux™ were included. They were prescribed two doses of 1 g Reditux™, at least 15 days apart, with continued stable doses of methotrexate. Efficacy and safety after 24 weeks relative to baseline was assessed using various health assessment variables. A total of 39 patients (mean age of 46 years; 67.5 % females) treated with Reditux™ were evaluated. Statistically significant differences were observed in mean changes of DAS28-CRP, DAS28-ESR, SDAI, HAQ and Patient Global Assessment scores from baseline to 24 weeks (p < 0.0001 for all). Average steroid use per week also significantly reduced at 24 weeks (p = 0.0002). There was no significant gender difference. Mean changes in SDAI, HAQ and Patient Global Assessment scores for patients on steroids were significantly different from those not on steroids (p < 0.05 for all). At 24 weeks, 97 % of patients achieved ACR20 response demonstrating the efficacy of Reditux™ treatment. The treatment was well tolerated by patients without any clinically relevant serious adverse events over 24 weeks. Though limited by number of patients and retrospective in nature, this analysis serves as a real-world evidence of efficacy and safety of Dr. Reddy’s rituximab (Reditux™) in the treatment of csDMARD-failed patients with RA over a 6-month period.
Similar content being viewed by others
References
Haraoui B, Bokarewa M, Kallmeyer I, Bykerk VP, RESET Investigators (2011) Safety and effectiveness of rituximab in patients with rheumatoid arthritis following an inadequate response to 1 prior tumor necrosis factor inhibitor: the RESET Trial. J Rheumatol 38(12):2548–2556
Rubbert-Roth A, Finckh A (2009) Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 11(Suppl 1):S1
Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T, Rituximab Consensus Expert Committee et al (2011) Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 70(6):909–920
Edwards JCW, Szczepanski L, Szechinski J et al (2004) Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med 350:2572–2581
Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC et al (2006) Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum 54:2793–2806
Emery P, Fleischmann R, Filipowicz-Sosnowska A, Schechtman J, Szczepanski L, Kavanaugh A, Racewicz AJ, van Vollenhoven RF, Li NF, Agarwal S, Hessey EW, Shaw TM, DANCER Study Group (2006) The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum 54(5):1390–1400
Lopez-Olivo MA, Tayar JH, Suarez-Almazor ME (2011) The updated guidelines on the use of rituximab in rheumatoid arthritis. Rheumatology (Oxford) 50(12):2153–2154
Bukhari M, Abernethy R, Deighton C et al (2011) BSR and BHPR guidelines on the use of rituximab in rheumatoid arthritis. Rheumatology (Oxford) 50(12):2311–2313
McGonagle D, Tan AL, Madden J, Taylor L, Emery P (2008) Rituximab use in everyday clinical practice as a first-line biologic therapy for the treatment of DMARD-resistant rheumatoid arthritis. Rheumatology 47:865–867
Rubbert-Roth A, Tak PP, Zerbini C, Tremblay JL, Carreño L, Armstrong G, Collinson N, Shaw TM, MIRROR Trial Investigators (2010) Efficacy and safety of various repeat treatment dosing regimens of rituximab in patients with active rheumatoid arthritis: results of a phase III randomized study (MIRROR). Rheumatology (Oxford) 49(9):1683–1693
Emery P, Deodhar A, Rigby WF, Isaacs JD, Combe B, Racewicz AJ, Latinis K, Abud-Mendoza C, Szczepanski LJ, Roschmann RA, Chen A, Armstrong GK, Douglass W, Tyrrell H (2010) Efficacy and safety of different doses and retreatment of rituximab: a randomised, placebo-controlled trial in patients who are biological naive with active rheumatoid arthritis and an inadequate response to methotrexate (study evaluating rituximab's efficacy in MTX iNadequate rEsponders (SERENE)). Ann Rheum Dis 69(9):1629–1635
Tak PP, Rigby WF, Rubbert-Roth A, Peterfy CG, van Vollenhoven RF, Stohl W, Hessey E, Chen A, Tyrrell H, Shaw TM, IMAGE Investigators (2011) Inhibition of joint damage and improved clinical outcomes with rituximab plus methotrexate in early active rheumatoid arthritis: the IMAGE trial. Ann Rheum Dis 70(1):39–46
Ramiro S, Gaujoux-Viala C, Nam JL, Smolen JS, Buch M, Gossec L, van der Heijde D, Winthrop K, Landewé R (2014) Safety of synthetic and biological DMARDs: a systematic literature review informing the 2013 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 73(3):529–535
Dr. Reddy’s launches Reditux™—monoclonal antibody treatment for nonHodgkin’s lymphoma. 2007 Apr 30. [Online]. [Accessed 20 April 2015]. Available from: http://www.drreddys.com/media/popups/apr30_2007.htm
Roy PS, John S, Karankal S et al (2013) Comparison of the efficacy and safety of rituximab (Mabthera™) and its biosimilar (Reditux™) in diffuse large B-cell lymphoma patients treated with chemo-immunotherapy: a retrospective analysis. Indian J Med Paediatr Oncol 34(4):292–298
Developing biosimilars in emerging markets: regulatory and clinical considerations. White paper. http://www.ppdi.com. Accessed Aug 2013
Smolen JS, Landewé R, Breedveld FC et al (2014) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 73(3):492–509
Biswas G, Parikh PM, Nair R et al (2006) Rituximab (anti-CD20 monoclonal antibody) in lymphoproliferative malignancies: Tata Memorial experience. J Assoc Physicians India 54:29–33
Acknowledgments
We thank Dr Hetal Shah from Arkus Research Pvt. Ltd., Ahmedabad, India, for her professional support in drafting this manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol for data collection and analysis received the Institutional Ethics Committee’s approval.
Disclosures
None.
Rights and permissions
About this article
Cite this article
Bhati, M., Bandyopadhyay, S. Efficacy and safety of an anti-CD20 monoclonal antibody (Reditux™) for the treatment of patients with moderate to severe rheumatoid arthritis following the failure of conventional synthetic disease-modifying anti-rheumatic drugs. Clin Rheumatol 35, 1931–1935 (2016). https://doi.org/10.1007/s10067-016-3332-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3332-8