Abstract
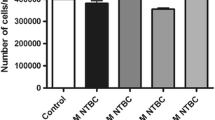
Alkaptonuria is a rare autosomal recessive condition resulting from inability to breakdown homogentisic acid (HGA), an intermediate in tyrosine degradation. The condition has a triad of clinical features, the most damaging of which is ochronotic osteoarthropathy. HGA is elevated from birth, but pigmentation takes many years. We hypothesise that interleukins play a role in initiation and progression of ochronotic osteoarthropathy. C20/A4 cells were cultured and maintained in 9-cm petri dishes containing either HGA at 0.33 mM, a single interleukin (IL-1β, IL-6 or IL-10) at 1 ng/ml or a combination of HGA and a single interleukin. Statistical analysis of pigment deposits and cell viability was performed using analysis of variance with Newman-Keuls post-test. All cultures containing HGA showed a significant increase in pigment deposition compared to control and IL cultures alone. The cultures containing HGA and IL-6 showed a significant increase in pigment deposits compared to HGA alone. The cell viability counts across all cultures on day 10 demonstrated a significant decrease in cultures containing HGA compared to those which did not. There was no significant difference between cultures containing just HGA or those combined with an interleukin. This work demonstrates a role for cytokines present in the joint(s) in the pigmentation process, particularly IL-6, and that the presence of HGA in joint tissues appears more detrimental to chondrocytes than the presence of any of the interleukins found in response to joint injury, trauma and osteoarthritis (OA). This further supports the evidence that the arthropathy in alkaptonuria is much more severe and rapidly progressing.






Similar content being viewed by others
References
Fisher AA, Davis MW (2004) Alkaptonuric ochronosis with aortic valve and joint replacements and femoral fracture. Clin Med Res 2:209–15
Fernandez-Canon JM, Granadino B, de Bernabe DBV, Renedo M, Fernandez-Ruiz E, Penalva MA et al (1996) The molecular basis of alkaptonuria. Nat Genet 14:19
La Du BN, Zannoni VG, Laster L, Seegmiller JE (1958) The nature of the defect in tyrosine metabolism in alcaptonuria. J Biol Chem 230:251
Mistry JB, Bukhari M, Taylor AM (2013) Alkaptonuria. Rare Dis 1, e27475. doi:10.4161/rdis.27475
Taylor AM, Wlodarski B, Prior IA, Wilson PJ, Jarvis JC, Ranganath LR et al (2010) Ultrastructural examination of tissue in a patient with alkaptonuric arthropathy reveals a distinct pattern of binding of ochronotic pigment. Rheumatology (Oxford) 49(7):1412–4
Taylor AM, Wilson PJ, Ingrams DR, Helliwell TR, Gallagher JA, Ranganath LR (2010) Calculi and intracellular ochronosis in the submandibular tissues from a patient with alkaptonuria. J Clin Pathol 63(2):186–8
Helliwell TR, Gallagher JA, Ranganath L (2008) Alkaptonuria—a review of surgical and autopsy pathology. Histopathology 53(5):503–12
Taylor AM, Boyde A, Wilson PJ, Jarvis JC, Davidson JS, Hunt JA et al (2011) The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 63(12):3887–96
Taylor AM, Boyde A, Davidson JS, Jarvis JC, Ranganath LR, Gallagher JA (2012) Identification of trabecular excrescences, novel microanatomical structures, present in bone in osteoarthropathies. Eur Cell Mater 23:300–9
Araki K, Sudo A, Hasegawa M, Uchida A (2009) Devastating ochronotic arthropathy with successful bilateral hip and knee arthroplasties. J Clin Rheumatol 15(3):138–40
Pettit SJ, Fisher M, Gallagher JA, Ranganath LR (2011) Cardiovascular manifestations of alkaptonuria. J Inherit Metab Dis 34(6):1177–81
Phornphutkul C, Introne WJ, Perry MB, Bernardini I, Murphey MD, Fitzpatrick DL et al (2002) Natural history of alkaptonuria. N Engl J Med 347(26):2111–21
Sridhar FK, Mukha RP, Kumar S, Kekre NS (2012) Lower urinary tract symptoms and prostatic calculi: a rare presentation of alkaptonuria. Indian J Urol 28(2):219–21
Krízek V (1971) Urolithiasis and prostatolithiasis in alcaptonuria with ochronosis. Int Urol Nephrol 3(3):245–50
Boyde A, Davis GR, Mills D, Zikmund T, Cox TM, Adams VL et al (2014) On fragmenting, densely mineralised acellular protrusions into articular cartilage and their possible role in osteoarthritis. J Anat 225(4):436–46
Ranganath LR, Jarvis JC, Gallagher JA (2013) Recent advances in management of alkaptonuria. J Clin Pathol 66(5):367–73
Lindstedt S, Holme E, Lock EA, Hjalmarson O, Strandvik B (1992) Treatment of hereditary tyrosinaemia type I by inhibition of 4-hydroxyphenylpyruvate dioxygenase. Lancet 340(8823):813–7
Lock EA, Ellis MK, Gaskin P, Robinson M, Auton TR, Provan WM et al (1998) From toxicological problem to therapeutic use: the discovery of the mode of action of 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC), its toxicology and development as a drug. J Inherit Metab Dis 21(5):498–506
Ranganath LR, Milan AM, Hughes AT, Dutton JJ, Fitzgerald R, Briggs MC et al (2014) Suitability of nitisinone in alkaptonuria 1 (SONIA 1): an international, multicentre, randomised, open-label, no-treatment controlled, parallel-group, dose–response study to investigate the effect of once daily nitisinone on 24-h urinary homogentisic acid excretion in patients with alkaptonuria after 4 weeks of treatment. Ann Rheum Dis. doi:10.1136/annrheumdis-2014-206033
Arnoux JB, Le Quan Sang KH, Brassier A, Grisel C, Servais A, Wippf J, et al (2015) Old treatments for new insights and strategies: proposed management in adults and children with alkaptonuria. J Inherit Metab Dis
Introne WJ, Perry MB, Troendle J, Tsilou E, Kayser MA, Suwannarat P et al (2011) A 3-year randomized therapeutic trial of nitisinone in alkaptonuria. Mol Genet Metab 103(4):307–14
Gertsman I, Barshop BA, Panyard-Davis J, Gangoiti JA, Nyhan WL (2015) Metabolic effects of increasing doses of nitisinone in the treatment of alkaptonuria. JIMD Rep
Lodh M, Kerketta JA (2013) Early diagnosis of co-existent ß-thalassemia and alkaptonuria. Indian J Hum Genet 19(2):259–61
Tinti L, Taylor AM, Santucci A, Wlodarski B, Wilson PJ, Jarvis JC et al (2011) Development of an in vitro model to investigate joint ochronosis in alkaptonuria. Rheumatology (Oxford) 50(2):271–7
Braconi D, Millucci L, Bernardini G, Santucci A (2015) Oxidative stress and mechanisms of ochronosis in alkaptonuria. Free Radic Biol Med. doi:10.1016/j.freeradbiomed.2015.02.021
Millucci L, Ghezzi L, Paccagnini E, Giorgetti G, Viti C, Braconi D et al (2014) Amyloidosis, inflammation, and oxidative stress in the heart of an alkaptonuric patient. Mediat Inflamm 2014:258471. doi:10.1155/2014/258471
Braconi D, Millucci L, Ghezzi L, Santucci A (2013) Redox proteomics gives insights into the role of oxidative stress in alkaptonuria. Expert Rev Proteomics 10(6):521–35
Spreafico A, Millucci L, Ghezzi L, Geminiani M, Braconi D, Amato L et al (2013) Antioxidants inhibit SAA formation and pro-inflammatory cytokine release in a human cell model of alkaptonuria. Rheumatology (Oxford) 52(9):1667–73
Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T et al (2002) Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis 61(Suppl 2):ii78–81
Pearle AD, Warren RF, Rodeo SA (2005) Basic science of articular cartilage and osteoarthritis. Clin Sports Med 24:1–12
Goldring MB (2000) Osteoarthritis and cartilage: the role of cytokines. Curr Rheumatol Rep 2:459–65
Goldring MB (2006) Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol 20:1003–25
Sovani S, Grogan SP (2013) Osteoarthritis: detection, pathophysiology, and current/future treatment strategies. Orthop Nurs 32:25–36
Goldring MB, Berenbaum F (2004) The regulation of chondrocyte function by proinflammatory mediators: prostaglandins and nitric oxide. Clin Orthop Relat Res 427:S37–46
Goldring SR, Goldring MB (2004) The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res 427:S27–36
Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11:224
Goldring MB, Otero M, Plumb DA, Dragomir C, Favero M, El Hachem K et al (2011) Roles of inflammatory and anabolic cytokines in cartilage metabolism: signals and multiple effectors converge upon MMP-13 regulation in osteoarthritis. Eur Cell Mater 21:202–20
Dayer JM (2004) The process of identifying and understanding cytokines: from basic studies to treating rheumatic diseases. Best Pract Res Clin Rheumatol 18:31–45
Loeser RF (2008) Molecular mechanisms of cartilage destruction in osteoarthritis. J Musculoskelet Neuronal Interact 8:303–6
Westacott CI, Sharif M (1996) Cytokines in osteoarthritis: mediators or markers of joint destruction? Semin Arthritis Rheum 25:254–72
Takaoka Y, Niwa S, Nagai H (1999) Interleukin-1B induces interleukin-6 production through the production of prostaglandin E2 in human osteoblasts, MG-63 cells. J Biochem 126:553–8
Alaaeddine N, Di Battista JA, Pelletier J, Kiansa K, Cloutier J, Martel-Pelletier J (1999) Inhibition of tumor necrosis factor & alpha-induced prostaglandin E2 production by the antiinflammatory cytokines interleukin-4, interleukin-10, and interleukin-13 in osteoarthritic synovial fibroblasts: distinct targeting in the signaling pathways. Arthritis Rheum 42:710–8
Lubberts E, Joosten LAB, Helsen MMA, Van Den Berg WB (1998) Regulatory role of interleukin 10 in joint inflammation and cartilage destruction in murine streptococcal cell wall (SCW) arthritis. More therapeutic benefit with IL-4/IL-10 combination therapy than with IL-10 treatment alone. Cytokine 10:361–9
Goldring MB, Birkhead JR, Suen LF et al (1994) Interleukin-1 beta-modulated gene expression in immortalized human chondrocytes. J Clin Invest 94:2307–2316
Blanco FJ, Lotz M (1995) IL-1-induced nitric oxide inhibits chondrocyte proliferation via PGE2. Exp Cell Res 218(1):319–25
López-Armada MJ, Caramés B, Lires-Deán M, Cillero-Pastor B, Ruiz-Romero C, Galdo F et al (2006) Cytokines, tumor necrosis factor-α and interleukin-1β, differentially regulate apoptosis in osteoarthritis cultured human chondrocytes. Osteoarthr Cartil 14(7):660–9
Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL et al (2014) Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther 16(3):R134
Bigoni M, Sacerdote P, Turati M, Franchi S, Gandolla M, Gaddi D, Moretti S, Munegato D, Augusti CA, Bresciani E, Omeljaniuk RJ, Locatelli V, Torsello A (2013) Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res 31:315–321
Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, Evans CH (1997) The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med 25:751–754
Marks PH, Donaldson ML (2005) Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy 21:1342–1347
Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ et al (2008) Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum 58:1707–1715
Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA (2008) Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum 58:744–753
Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM et al (2009) Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum 60(7):2037–45
Stannus O, Jones G, Cicuttini F, Parameswaran V, Quinn S, Burgess J et al (2010) Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr Cartil 18(11):1441–7
Helmark IC, Mikkelsen UR, Børglum J, Rothe A, Petersen MC, Andersen O et al (2010) Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther 12(4):R126
Kaneva MK, Kerrigan MJ, Grieco P, Curley GP, Locke IC, Getting SJ (2012) Chondroprotective and anti-inflammatory role of melanocortin peptides in TNF-α activated human C-20/A4 chondrocytes. Br J Pharmacol 167(1):67–79
Bálint G, Szebenyi B (2000) Hereditary disorders mimicking and/or causing premature osteoarthritis. Baillieres Best Pract Res Clin Rheumatol 14:219–50
Lagier R (2006) Ochronotic arthropathy, an approach to osteoarthritis bone remodelling. Rheumatol Int 26:561–4
Braconi D, Laschi M, Taylor AM, Bernardini G, Spreafico A, Tinti L et al (2010) Proteomic and redox-proteomic evaluation of homogentisic acid and ascorbic acid effects on human articular chondrocytes. J Cell Biochem 111(4):922–32
Mathy-Hartert M, Hogge L, Sanchez C, Deby-Dupont G, Crielaard JM, Henrotin Y (2008) Interleukin-1beta and interleukin-6 disturb the antioxidant enzyme system in bovine chondrocytes: a possible explanation for oxidative stress generation. Osteoarthr Cartil 16(7):756–63
Albataineh E, Al-Sabou M, Al-Sarayreh S, Al-Tarawneh I, Alnawaiseh N (2014) Levels of pro-inflammatory mediators CRP, IL-1β and IL-6 in alkaptonuria patients. J Biol Life Sci 6(1):37–46
Mistry JB, Jackson DJ, Bukhari M, Taylor AM (2015) Osteoarticular cells tolerate short-term exposure to nitisinone—implications in alkaptonuria. Clin Rheumatol
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Funding
The authors would like to thank the AKU Society, the Rosetrees Trust and University Hospitals Morecambe Bay NHS Foundation Trust for funding.
Additional information
Key messages
HGA is more detrimental to chondrocyte viability than interleukins; hence, alkaptonuria arthropathy is more severe.
IL-6 appears to be involved in increasing ochronotic pigmentation in alkaptonuria
IL-1β and IL-10 do not appear to affect ochronotic pigmentation in alkaptonuria.
Rights and permissions
About this article
Cite this article
Mistry, J.B., Jackson, D.J., Bukhari, M. et al. A role for interleukins in ochronosis in a chondrocyte in vitro model of alkaptonuria. Clin Rheumatol 35, 1849–1856 (2016). https://doi.org/10.1007/s10067-015-3091-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-015-3091-y