Abstract
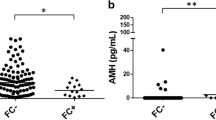
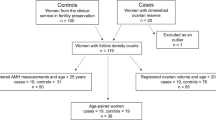
This study aims to assess ovarian reserve markers in Behçet's disease (BD) patients. Ten BD and 22 healthy controls were evaluated for ovarian reserve by examining the levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), estradiol, inhibin B, total morning testosterone, prolactin, thyroid-stimulating hormone (TSH), and antral follicle count. Anti-Müllerian hormone (AMH) was measured using two different enzyme-linked immunosorbent assay (ELISA) kits. Demographic data, menstrual abnormalities, disease parameters, and treatments were also analyzed. The median current age was similar in BD patients and controls (34 (20–40) vs. 31.3 (20–42) years, p = 0.33). A positive correlation was observed between the AMH Gen II ELISA and AMH/MISAnshLabs ELISA assays in the BD patients (r = +0.98; p < 0.0001) and healthy controls (r = +0.93; p < 0.0001). The mean AMH by Gen II (0.93 ± 0.8 vs. 2.59 ± 1.8 ng/mL, p = 0.01) and AMH/MIS AnshLabs ELISA (1.07 ± 0.86 vs. 2.51 ± 1.8 ng/mL, p = 0.02) were significantly reduced in the BD patients versus controls. A trend of decreased AMH (<1.0 ng/mL) was observed in BD patients compared to that in the controls (50 vs. 19 %, p = 0.09) using either kits. The mean FSH was significantly higher in the BD patients compared to that in the controls (9.1 ± 3.6 vs. 6.5 ± 2.7, p = 0.04). No differences were found for the other ovarian parameters in both groups (p > 0.05). Current disease activity was only observed in BD patients with a low AMH level; however, there was no statistical significance (40 vs. 0 %, p = 0.44). Cyclophosphamide use was reported in only one patient with a low AMH and high FSH level. The present study was the first to suggest that BD patients may have diminished ovarian reserve. The contribution of disease activity remains to be determined.
Similar content being viewed by others
References
Aikawa NE, Gonçalves C, Silva CA, Gonçalves C, Bonfá E, de Carvalho JF (2001) Late response to anti-TNF-α therapy in refractory mucocutaneous lesions of Behçet’s disease. Rheumatol Int 31:1097–1099
Silva CA, Brunner HI (2007) Gonadal functioning and preservation of reproductive fitness with juvenile systemic lupus erythematosus. Lupus 16:593–599
Silva CA, Yamakami LY, Aikawa NE, Araujo DB, Carvalho JF, Bonfá E (2014) Autoimmune primary ovarian insufficiency. Autoimmun Rev 13:427–430
Silva CA, Bonfa E, Østensen M (2010) Maintenance of fertility in patients with rheumatic diseases needing antiinflammatory and immunosuppressive drugs. Arthritis Care Res (Hoboken) 62:1682–1690
Ostensen M, Brucato A, Carp H et al (2011) Pregnancy and reproduction in autoimmune rheumatic diseases. Rheumatology (Oxford) 50:657–664
Aikawa NE, Sallum AM, Pereira RM et al (2012) Subclinical impairment of ovarian reserve in juvenile systemic lupus erythematosus after cyclophosphamide therapy. Clin Exp Rheumatol 30:445–449
Silva CA, Leal MM, Leone C et al (2002) Gonadal function in adolescents and young women with juvenile systemic lupus erythematosus. Lupus 11:419–425
Silva CA, Hilário MO, Febrônio MV et al (2007) Risk factors for amenorrhea in juvenile systemic lupus erythematosus (JSLE): a Brazilian multicentre cohort study. Lupus 16:531–536
Medeiros P, Febrônio M, Bonfá E, Borba E, Takiuti A, Silva CA (2009) Menstrual and hormonal alterations in juvenile systemic lupus erythematosus. Lupus 18:38–43
Aikawa NE, Sallum AM, Leal MM, Bonfá E, Pereira RM, Silva CA (2010) Menstrual and hormonal alterations in juvenile dermatomyositis. Clin Exp Rheumatol 28:571–575
Uzunaslan D, Saygin C, Hatemi G, Tascilar K, Yazici H (2014) No appreciable decrease in fertility in Behcet’s syndrome. Rheumatology (Oxford) 53:828–833
Çil AP, Karabulut AA, Koçak M (2010) Assessment of ovarian stromal artery Doppler characteristics and serum hormone levels in patients with Behçet disease. Diagn Interv Radiol 16:288–292
(1990) Criteria for diagnosis of Behçet’s disease. International Study Group for Behçet’s Disease. Lancet 335:1078-1080
Satwik R, Kochhar M, Gupta SM, Majumdar A (2012) Anti-Mullerian hormone cut-off values for predicting poor ovarian response to exogenous ovarian stimulation in in-vitro fertilization. J Hum Reprod Sci 5:206–212
Hendriks DJ, Kwee J, Mol BW, te Velde ER, Broekmans FJ (2007) Ultrasonography as a tool for the prediction of outcome in IVF patients: a comparative meta-analysis of ovarian volume and antral follicle count. Fertil Steril 87:764–775
Neves FS, Moraes JC, Kowalski SC, Goldenstein-Schainberg C, Lage LV, Gonçalves CR (2007) Cross-cultural adaptation of the Behçet’s Disease Current Activity Form (BDCAF) to Brazilian Portuguese language. Clin Rheumatol 26:1263–1267
Freour T, Masson D, Mirallie S et al (2008) Active smoking compromises IVF outcome and affects ovarian reserve. Reprod Biomed Online 16:96–102
Clark CA, Laskin CA, Cadesky K (2014) Anti-Mullerian hormone: reality check. Hum Reprod 29:184–185
Practice Committee of the American Society for Reproductive Medicine (2012) Testing and interpreting measures of ovarian reserve: a committee opinion. Fertil Steril 98:1407–1415
Seifer DB, Golub ET, Lambert-Messerlian G et al (2009) Variations in serum Müllerian inhibiting substance between white, black, and Hispanic women. Fertil Steril 92:1674–1678
Bleil ME, Gregorich SE, Adler NE, Sternfeld B, Rosen MP, Cedars MI (2014) Race/ethnic disparities in reproductive age: an examination of ovarian reserve estimates across four race/ethnic groups of healthy, regularly cycling women. Fertil Steril 101:199–207
Silva CA, Cocuzza M, Borba EF, Bonfá E (2012) Cutting-edge issues in autoimmune orchitis. Clin Rev Allergy Immunol 42:256–263
Silva CA, Hallak J, Pasqualotto FF, Barba MF, Saito MI, Kiss MH (2002) Gonadal function in male adolescents and young males with juvenile onset systemic lupus erythematosus. J Rheumatol 29:2000–2005
Soares PM, Borba EF, Bonfa E, Hallak J, Correa AL, Silva CA (2007) Gonad evaluation in male systemic lupus erythematosus. Arthritis Rheum 56:2352–2361
Suehiro RM, Borba EF, Bonfa E et al (2008) Testicular Sertoli cell function in male systemic lupus erythematosus. Rheumatology 47:1692–1697
Moraes AJ, Pereira RM, Cocuzza M, Casemiro R, Saito O, Silva CA (2008) Minor sperm abnormalities in young male post-pubertal patients with juvenile dermatomyositis. Braz J Med Biol Res 41:1142–1147
Morales AJ, Bonfa E, Cocuzza M, Borges CT, Saito O, Silva CA (2010) Gonad evaluation in male dermatomyositis. A pilot study. Clin Exp Rheumatol 28:441–442
Acknowledgments
This study was supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP# 11/12471-2 to CAS), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ #301411/2009-3 to EB and #302724/2011-7 to CAS), Federico Foundation (to EB and CAS), and Núcleo de Apoio à Pesquisa “Saúde da Criança e do Adolescente” da USP (NAP-CriAd-SP) to CAS. We thank Elaine P. Leon and Vilma T. Viana for their technical support and Dr. Ulysses Doria Filho for the statistical analysis.
Disclosures
Andrea R.S. Mont’Alverne, Lucas Y.S. Yamakami, Célio R. Gonçalves, Edmund C. Baracat, Eloisa Bonfá, and Clovis A. Silva have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mont’Alverne, A.R.S., Yamakami, L.Y.S., Gonçalves, C.R. et al. Diminished ovarian reserve in Behçet’s disease patients. Clin Rheumatol 34, 179–183 (2015). https://doi.org/10.1007/s10067-014-2680-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2680-5