Abstract
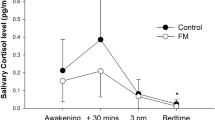
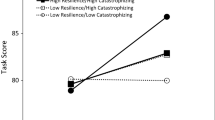
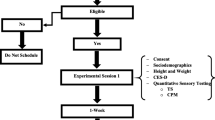
The present study aims at studying interactions between cognitive performance and conditioned pain modulation in patients with chronic whiplash-associated disorders (WAD) and healthy controls. In addition, the relation between cortisol concentrations and cognitive performance will be studied in patients with chronic WAD. Thirty-one subjects, 16 healthy subjects and 15 patients with chronic WAD, were enrolled and subjected to several self-report and physiological measures. Self-report measures encompassed pain rating during a procedure evaluating conditioned pain modulation. Afterward, they were subjected to physiological measures, which are cognitive tests (Stroop task, psychomotor vigilance task, and operation span task) preceded and followed by salivary cortisol concentration measurements. Chronic WAD patients performed worse in recall at the operation span task and presented longer reaction times at the psychomotor vigilance task and at the Stroop task when sleep-related words were shown (p < .05). Conditioned pain modulation and cortisol concentrations were not significantly different between patients and controls (p > .05). Only in the healthy subjects, conditioned pain modulation and baseline cortisol concentrations were correlated to cognitive performance (p < .05). This is the first study addressing the relation between pain inhibition and cognitive performance in chronic WAD. We did not reveal impaired pain inhibition but did reveal cognitive dysfunctions in patients with chronic WAD. In healthy subjects, pain inhibition was related to cognitive performance but not in the patient group.

Similar content being viewed by others
References
Sterling M, Treleaven J, Edwards S, Jull G (2002) Pressure pain thresholds in chronic whiplash associated disorder: further evidence of altered central pain processing. J Musculoskelet Pain 10(3):69–81
Curatolo M, Petersen-Felix S, Arendt-Nielsen L, Giani C, Zbinden AM, Radanov BP (2001) Central hypersensitivity in chronic pain after whiplash injury. Clin J Pain 17(4):306–315
Sterling M, Jull G, Vicenzino B, Kenardy J (2003) Sensory hypersensitivity occurs soon after whiplash injury and is associated with poor recovery. Pain 104(3):509–517
Jull G, Sterling M, Kenardy J, Beller E (2007) Does the presence of sensory hypersensitivity influence outcomes of physical rehabilitation for chronic whiplash?–A preliminary RCT. Pain 129(1–2):28–34
Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL et al (1990) The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33(2):160–172
Nederhof E, Lemmink KA, Visscher C, Meeusen R, Mulder T (2006) Psychomotor speed: possibly a new marker for overtraining syndrome. Sports Med (Auckland, NZ 36(10):817–828
Spitzer WO, Skovron ML, Salmi LR, Cassidy JD, Duranceau J, Suissa S et al (1995) Scientific monograph of the Quebec Task Force on whiplash-associated disorders: redefining "whiplash" and its management. Spine (Phila Pa 1976) 20(8 Suppl):1S–73S
Schmand B, Lindeboom J, Schagen S, Heijt R, Koene T, Hamburger HL (1998) Cognitive complaints in patients after whiplash injury: the impact of malingering. J Neurol Neurosurg Psychiatry 64(3):339–343
Weiner DK, Rudy TE, Morrow L, Slaboda J, Lieber S (2006) The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med (Malden, Mass 7(1):60–70
Moriarty O, McGuire BE, Finn DP (2011) The effect of pain on cognitive function: a review of clinical and preclinical research. Prog Neurobiol 93(3):385–404
Nijs J, Van Houdenhove B, Oostendorp RA (2010) Recognition of central sensitization in patients with musculoskeletal pain: application of pain neurophysiology in manual therapy practice. Man Ther 15(2):135–141
Borenstein P, Rosenfeld M, Gunnarsson R (2010) Cognitive symptoms, cervical range of motion and pain as prognostic factors after whiplash trauma. Acta Neurol Scand 122(4):278–285
Linnman C, Appel L, Soderlund A, Frans O, Engler H, Furmark T et al (2009) Chronic whiplash symptoms are related to altered regional cerebral blood flow in the resting state. Eur J Pain (London, England) 13(1):65–70
Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T (2008) Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain 131(Pt 12):3222–3231
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55(3):377–391
Kosek E, Hansson P (1997) Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 70(1):41–51
Lautenbacher S, Rollman GB (1997) Possible deficiencies of pain modulation in fibromyalgia. Clin J pain 13(3):189–196
Julien N, Goffaux P, Arsenault P, Marchand S (2005) Widespread pain in fibromyalgia is related to a deficit of endogenous pain inhibition. Pain 114(1–2):295–302
Daenen L, Nijs J, Roussel N, Wouters K, Van Loo M, Cras P (2011) Inefficient diffuse noxious inhibitory controls in chronic whiplash associated disorders: an experimental study. Eur J Pain 1(Suppl 5):129
Tytherleigh MY, Vedhara K, Lightman SL (2004) Mineralocorticoid and glucocorticoid receptors and their differential effects on memory performance in people with Addison's disease. Psychoneuroendocrinology 29(6):712–723
Gaab J, Baumann S, Budnoik A, Gmunder H, Hottinger N, Ehlert U (2005) Reduced reactivity and enhanced negative feedback sensitivity of the hypothalamus-pituitary-adrenal axis in chronic whiplash-associated disorder. Pain 119(1–3):219–224
Tipper SP (1985) The negative priming effect: inhibitory priming by ignored objects. Q J Exp Psychol A Human Exp psychol 37(4):571–590
Dinges DF, Powell JW (1985) Microcomputer analyses of performance on a portable, simple visual Rt task during sustained operations. Behav Res Meth Instr 17(6):652–655
Wilkinson RT, Houghton D (1982) Field test of arousal: a portable reaction timer with data storage. Human factors 24(4):487–493
Conway AR, Engle RW (1996) Individual differences in working memory capacity: more evidence for a general capacity theory. Memory 4(6):577–590
Cathcart S, Winefield AH, Rolan P, Lushington K (2009) Reliability of temporal summation and diffuse noxious inhibitory control. Pain Res Manag 14(6):433–438
Meeus M, Ickmans K, De Clerck LS, Moorkens G, Hans G, Grosemans S et al (2011) Serotonergic descending inhibition in chronic pain: design, preliminary results and early cessation of a randomized controlled trial. In vivo (Athens, Greece) 25(6):1019–1025
Vanderweeen L, Oostendorp RA, Vaes P, Duquet W (1996) Pressure algometry in manual therapy. Man Ther 1(5):258–265
Kosek E, Ekholm J, Hansson P (1999) Pressure pain thresholds in different tissues in one body region. The influence of skin sensitivity in pressure algometry. Scand J Rehabil Med 31(2):89–93
Meeus M, Ickmans K, Oderkerk J, Struyf F, Hermans L, De Clerck LS, et al. (2013) Does acetaminophen activate endogenous pain inhibition in chronic fatigue syndrome/fibromyalgia and rheumatoid arthritis? A double-blind randomized controlled cross-over trial. Pain Physician (accepted for publication). Pain Physician 16(2):E61–70
Staud R, Robinson ME, Price DD (2007) Temporal summation of second pain and its maintenance are useful for characterizing widespread central sensitization of fibromyalgia patients. J Pain 8(11):893–901
Fernandez-Carnero J, Fernandez-de-Las-Penas C, de la Llave-Rincon AI, Ge HY, Arendt-Nielsen L (2009) Widespread mechanical pain hypersensitivity as sign of central sensitization in unilateral epicondylalgia: a blinded, controlled study. Clin J Pain 25(7):555–561
Meeus M, Nijs J, Huybrechts S, Truijen S (2010) Evidence for generalized hyperalgesia in chronic fatigue syndrome: a case control study. Clin Rheumatol 29(4):393–398
Aardal E, Holm AC (1995) Cortisol in saliva–reference ranges and relation to cortisol in serum. Eur J Clin Chem Clin Biochem 33(12):927–932
Kirschbaum C, Hellhammer DH (1994) Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology 19(4):313–333
Vining RF, McGinley RA (1987) The measurement of hormones in saliva: possibilities and pitfalls. J Steroid Biochem 27(1–3):81–94
Vining RF, McGinley RA, Maksvytis JJ, Ho KY (1983) Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem 20(Pt 6):329–335
Antepohl W, Kiviloog L, Andersson J, Gerdle B (2003) Cognitive impairment in patients with chronic whiplash-associated disorder–a matched control study. NeuroRehabilitation 18(4):307–315
Lim J, Dinges DF (2008) Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129:305–322
Lee IS, Bardwell WA, Ancoli-Israel S, Dimsdale JE (2010) Number of lapses during the psychomotor vigilance task as an objective measure of fatigue. J Clin Sleep Med JCSM Fff Publ Am Acad Sleep Med 6(2):163–168
Blokhorst M, Swinkels M, Lof O, Lousberg R, Zilvold G (2002) The influence of "State" related factors on focused attention following whiplash associated disorder. J Clin Exp Neuropsychol 24(4):471–478
Guez M, Brannstrom R, Nyberg L, Toolanen G, Hildingsson C (2005) Neuropsychological functioning and MMPI-2 profiles in chronic neck pain: a comparison of whiplash and non-traumatic groups. J Clin Exp Neuropsychol 27(2):151–163
Kirschbaum C, Wolf OT, May M, Wippich W, Hellhammer DH (1996) Stress- and treatment-induced elevations of cortisol levels associated with impaired declarative memory in healthy adults. Life sciences 58(17):1475–1483
Lundberg U (2005) Stress hormones in health and illness: the roles of work and gender. Psychoneuroendocrinology 30(10):1017–1021
Acknowledgments
Mira Meeus is an awardee of the 2012 early research career grant of the International Association for the Study of Pain (IASP), funded by the Scan│Design Foundation by INGER & JENS BRUUN. Kelly Ickmans is a research fellow of ME Research UK.The authors are grateful to Johan Schiettecatte for kindly providing his expertise on cortisol analyses and to Niko De Temmerman and Tinne Boey en Wouter Rosseels for assistance in data collection.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meeus, M., Van Oosterwijck, J., Ickmans, K. et al. Interrelationships between pain processing, cortisol and cognitive performance in chronic whiplash-associated disorders. Clin Rheumatol 34, 545–553 (2015). https://doi.org/10.1007/s10067-013-2446-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2446-5