Abstract
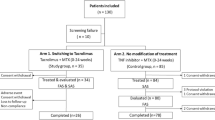
To retrospectively evaluate the efficacy and safety of combination therapy with tacrolimus (TAC) and other disease-modifying antirheumatic drugs (DMARDs). One hundred fifteen rheumatoid arthritis (RA) patients treated with tacrolimus were enrolled in this retrospective analysis. We collected clinical information, including patient background, treatment efficacy (evaluated using the DAS score), and adverse events observed. Multiple logistic regression analysis was conducted to analyze factors contributing to clinical response and adverse effects. The disease activity score of 28 joints (DAS28) improved significantly at 24 weeks, and continuation rate at 1 year was 57.9%. There was no difference in continuation rate between different DMARD combinations, and not only methotrexate (MTX) but also bucillamine (BUC) and salazosulfapyridine (SSZ) were effective combination partners with TAC. No serious adverse events were observed, and no different inefficacy or safety was observed between non-elderly (<65 years old) and elderly (≥65 years old) RA patients. By conducting multiple logistic regression analysis, combination therapy with MTX and TAC, the number of baseline DMARDs (specifically, ≥3), and old age were identified as risk factors for adverse events. Our findings indicate that TAC is a valuable DMARD for second-line combination therapy in RA.




Similar content being viewed by others
References
Furst D, Saag K, Fleischmann M, Sherrer Y, Block J, Schnitzer T et al (2002) Efficacy of tacrolimus in rheumatoid arthritis patients who have been treated unsuccessfully with methotrexate: a six-month, double-blind, randomized, dose-ranging study. Arthritis Rheum 46:2020–2028
Yocum D, Furst D, Kaine J, Baldassare A, Stevenson J, Borton M et al (2003) Efficacy and safety of tacrolimus in patients with rheumatoid arthritis: a double-blind trial. Arthritis Rheum 48:3328–3337
Yocum D, Furst D, Bensen W, Burch F, Borton M, Mengle-Gaw L et al (2004) Safety of tacrolimus in patients with rheumatoid arthritis: long-term experience. Rheumatology (Oxford) 43:992–999
Kawai S, Hashimoto H, Kondo H, Murayama T, Kiuchi T, Abe T (2006) Comparison of tacrolimus and mizoribine in a randomized, double-blind controlled study in patients with rheumatoid arthritis. J Rheumatol 33:2153–2161
Kawai S, Yamamoto K (2006) Safety of tacrolimus, an immunosuppressive agent, in the treatment of rheumatoid arthritis in elderly patients. Rheumatology (Oxford) 45:441–444
Kawai S, Tanaka K, Ohno I, Utsunomiya K, Seino Y (2008) Safety of long-term tacrolimus therapy for rheumatoid arthritis: an open-label, uncontrolled study in non-elderly patients. Mod Rheumatol 18:345–353
Kondo H, Abe T, Hashimoto H, Uchida S, Irimajiri S, Hara M et al (2004) Efficacy and safety of tacrolimus (FK506) in treatment of rheumatoid arthritis: a randomized, double blind, placebo controlled dose-finding study. J Rheumatol 31:243–251
Suzuki K, Kameda H, Amano K, Nagasawa H, Takei H, Sekiguchi N et al (2009) Single center prospective study of tacrolimus efficacy and safety in treatment of rheumatoid arthritis. Rheumatol Int 29:431–436
Kremer J, Habros J, Kolba K, Kaine J, Borton M, Mengle-Gaw L et al (2003) Tacrolimus in rheumatoid arthritis patients receiving concomitant methotrexate: a six-month, open-label study. Arthritis Rheum 48:2763–2768
Morita Y, Sasae Y, Sakuta T, Satoh M, Sasaki T, Kashihara N (2008) Efficacy of low-dose tacrolimus added to methotrexate in patients with rheumatoid arthritis in Japan: a retrospective study. Mod Rheumatol 18:379–384
Arnett F, Edworthy S, Bloch D, McShane D, Fries J, Cooper N et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Steinbrocker O, Traeger CH, Batterman RC (1949) Therapeutic criteria in rheumatoid arthritis. J Am Med Assoc 140:659–662
O'Dell J, Leff R, Paulsen G, Haire C, Mallek J, Eckhoff P et al (2002) Treatment of rheumatoid arthritis with methotrexate and hydroxychloroquine, methotrexate and sulfasalazine, or a combination of the three medications: results of a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum 46:1164–1170
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogasawara, M., Tamura, N., Kageyama, M. et al. Single-center, retrospective analysis of efficacy and safety of tacrolimus as a second-line DMARD in combination therapy and the risk factors contributing to adverse events in 115 patients with rheumatoid arthritis. Clin Rheumatol 31, 251–257 (2012). https://doi.org/10.1007/s10067-011-1810-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1810-6