Abstract
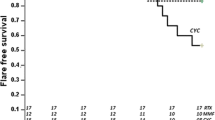
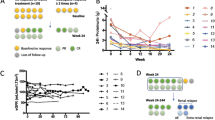
The objective of the current work is to report our preliminary experience with the mizoribine (MZR) intermittent pulse protocol for induction therapy for newly diagnosed pediatric-onset systemic lupus erythematosus (SLE). Five consecutive patients who were newly diagnosed as having SLE with biopsy-proven lupus nephritis were recruited for an open-label trial of prednisolone (PDN) and MZR intermittent pulse therapy (10 mg/kg for 2 days of the week for 12 months). Data on the renal response and serologic lupus activity were collected prospectively. The baseline characteristics of the patients were: mean age, 11 years; urinary protein/creatinine ratio (U-prot./cre.), 0.99 ± 0.91; serum complement hemolytic activity (CH50), 10.6 ± 1.3 (normal, 23–46 U/ml); serum anti-dsDNA antibody titer, 258.6 ± 125.5 IU/ml (normal, <12.0 IU/ml); serum creatinine, 0.5 ± 0.1 mg/dl; European Consensus Lupus Activity Measurement index (ECLAM), 7.4 ± 1.1. The primary endpoint was the interval until the development of a flare of SLE. Despite gradual tapering of the PDN dose, significant improvement as compared to the baseline values was observed in all the parameters examined at 3, 6, and 12 months of treatment. After 12 months therapy, complete response was achieved in all of the patients, except for 1 patient who showed poor drug compliance. In two patients who had severe lupus nephritis at the first renal biopsy, marked histologic improvement was confirmed at the second renal biopsy. No serious adverse effects were observed. We believe that the MZR pulse protocol combined with PDN for induction therapy may be the treatment of choice in selected young patients with SLE. Further studies to confirm the long-term efficacy and safety of our current protocol in larger numbers of patients are, however, needed.
Similar content being viewed by others
References
Lai KN, Tang SC, Mok CC (2005) Treatment for lupus nephritis: a revisit. Nephrology (Carlton) 10:180–188
Yokota S (2002) Mizoribine: mode of action and effects in clinical use. Pediatr Int 44:196–198
Hirayama K, Kobayashi M, Hashimoto Y et al (2004) Treatment with the purine synthesis inhibitor mizoribine for ANCA-associated renal vasculitis. Am J Kidney Dis 44:57–63
Tanaka H, Tsugawa K, Tsuruga K et al (2004) Mizoribine for the treatment of lupus nephritis in children and adolescents. Clin Nephrol 62:412–417
Yumura W, Suganuma K, Uchida K et al (2005) Effects of long-term treatment with mizoribine in patients with proliferative lupus nephritis. Clin Nephrol 64:28–34
Sonda K, Takahashi K, Tanabe K et al (1996) Clinical pharmacokinetic study of mizoribine in renal transplantation patients. Transplant Proc 28:3643–3648
Takahashi S, Wakui H, Gustafsson JA et al (2000) Functional interaction of the immunosuppressant mizoribine with the 14-3-3 protein. Biochem Biophys Res Commun 274:87–92
Tanaka H, Suzuki K, Nakahata T et al (2003) Mizoribine oral pulse therapy for patients with disease flare of lupus nephritis. Clin Nephrol 60:390–394
Tanaka H, Tsugawa K, Suzuki K et al (2006) Long-term mizoribine intermittent pulse therapy for young patients with flare of lupus nephritis. Pediatr Nephrol 21:962–966
Kawasaki Y, Hosoya M, Kobayashi S et al (2005) Oral mizoribine pulse therapy for patients with steroid-resistant and frequently relapsing steroid-dependent nephrotic syndrome. Nephrol Dial Transplant 20:2243–2247
Tanaka H, Tsugawa K, Nakahata T et al (2005) Mizoribine pulse therapy for a pediatric patient with steroid-resistant nephrotic syndrome. Tohoku J Exp Med 205:87–91
Tanaka H, Tateyama T, Waga S (2001) Metylprednisolone pulse therapy in Japanese children with severe lupus nephritis. Pediatr Nephrol 16:817–819
Mosca M, Bencivelli W, Vitali C et al (2000) The validity of the ECLAM index for the retrospective evaluation of disease activity in systemic lupus erythematosus. Lupus 9:445–450
Tanaka H, Oki Es, Tsugawa K et al (2007) Long-term intermittent pulse therapy with mizoribine attenuates histologic progression in young patients with severe lupus nephritis: report of two patients. Nephrology 12 (in press)
Yumura W, Uchida K, Kawashima A et al (1999) Evaluation of plasma concentration of mizoribine as an immunosuppressive agent in lupus nephritis patients. Jin To Toseki 47:705–708 (in Japanese)
Yang LY, Chen WP, Lin CY (1994) Lupus nephritis in children—a review of 167 patients. Pediatrics 94:335–340
Lehman TJ, Onel K (2000) Intermittent intravenous cyclophosphamide arrests progression of the renal chronicity index in childhood systemic lupus erythematosus. J Pediatr 136:243–247
Niaudet P (2000) Treatment of lupus nephritis in children. Pediatr Nephrol 14:158–166
Acknowledgment
The authors would like to thank Asahi-Kasei Pharm, Tokyo for conducting the measurements of the blood levels of MZR.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, H., Tsugawa, K., Oki, E. et al. Mizoribine intermittent pulse protocol for induction therapy for systemic lupus erythematosus in children: an open-label pilot study with five newly diagnosed patients. Clin Rheumatol 27, 85–89 (2008). https://doi.org/10.1007/s10067-007-0635-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-007-0635-9