Abstract
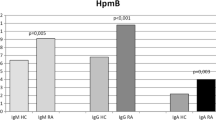
It has been suggested that Proteus infection may be involved in the pathogenesis of rheumatoid arthritis (RA). Bacterial and peptide immune responses in patients with RA and other control subjects were investigated in two geographically different populations. Serum samples from Finnish patients with early (n=72) and advanced (n=27) RA and 30 Finnish healthy controls, as well as from Japanese RA patients from two different locations: Tokyo (n=30) and Otsu (n=30), 18 patients with systemic lupus erythematosus (SLE) and 23 Japanese healthy controls were all screened for the total, and class-specific (IgG, IgA and IgM) antibodies against Proteus mirabilis, Escherichia coli and Serratia marcescens by indirect immunofluorescence assay. These samples were also tested for the determination of levels of isotypic antibodies against the shared epitope involving 16-mer synthetic peptides containing the EQRRAA or ESSRAL sequences and compared to scrambled control peptide by using an enzyme-labeled immunosorbent assay method. Significantly elevated levels of IgG and IgM antibodies to P. mirabilis and antibodies against both EQRRAA and ESSRAL peptides were detected in sera of Finnish patients with early and advanced RA, and in Japanese patients from Otsu or Tokyo compared to their corresponding control groups. In contrast, no difference either in the total or in any of the isotypic antibodies were observed between these groups when serum samples were screened against each of E. coli and S. marcescens or against the control peptide. Furthermore, there was a significant correlation between the antibody levels against Proteus bacteria only and both EQRRAA and ESRRAL peptides. Our findings support the possibility for specific involvement of P. mirabilis in the etiopathogenesis of RA even in early cases.





Similar content being viewed by others
Abbreviations
- ELISA:
-
Enzyme linked immunosorbent assay
- IIF:
-
Immunofluorescence
- RA:
-
Rheumatoid arthritis
- SLE:
-
Systemic lupus erythematosus
References
Carty SM, Snowden N, Silman AJ (2003) Should infection still be considered as the most likely triggering factor for rheumatoid arthritis? J Rheumatol 30:425–429
Stastny P (1976) Mixed lymphocyte cultures in rheumatoid arthritis. J Clin Invest 57:1148–1157
Nakai Y, Wakisaka A, Aizawa M, Itakura K, Nakai H, Ohashi A (1981) HLA and rheumatoid arthritis in Japanese. Arthritis Rheum 24:722–725
Ohta N, Nishimura YK, Tanimoto K et al. (1982) Association between HLA and Japanese patients with rheumatoid arthritis. Hum Immunol 5:123–132
Gorodezky C, Lavalle C, Castro-Escobar LE, Miranda-Limon JM, Escobar-Gutierrez A (1981) HLA antigens in Mexican patients with adult rheumatoid arthritis. Arthritis Rheum 24:976–977
Alarcon GS, Koopman WJ, Acton RT, Barger BO (1983) DR antigen distribution in blacks with rheumatoid arthritis. J Rheumatol 10:579–583
Woodrow JC, Nichol FE, Zaphiropoulos G (1981) DR antigens and rheumatoid arthritis: a study of two populations. Br Med J 283:1287–1288
Schiff B, Mizrachi Y, Orgad S, Yaron M, Gazit E (1982) Association of HLA-Aw31 and HLA-DR1 with adult rheumatoid arthritis. Ann Rheum Dis 41:403–404
Willkens RF, Hansen JA, Malmgren JA, Nisperos B, Mickelson EM, Watson MA (1982) HLA antigens in Yakima Indians with rheumatoid arthritis. Lack of association with HLA-Dw4 and HLA-DR4. Arthritis Rheum 25:1435–1439
Watanabe Y, Tokunaga K, Matsuki K et al. (1989) Putative amino acid sequence of HLA-DRß chain contributing to rheumatoid arthritis susceptibility. J Exp Med 169:2263–2268
Wallin J, Hillert J, Olerup O, Carlsson B, Strom H (1991) Association of rheumatoid arthritis with a dominant DR1/Dw4/Dw14 sequence motif, but not with T cell receptor beta chain gene alleles or haplotypes. Arthritis Rheum 34:1416–1424
Gregersen PK, Silver J, Winchester RJ (1987) The shared epitope hypothesis: An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 30:1205–1213
Tuokko J, Nejentsev S, Luukkainen R, Toivanen A, Ilonen J (2001) HLA haplotype analysis in Finnish patients with rheumatoid arthritis. Arthritis Rheum 44:315–322
De Vries N, Tijssen H, Jarvinen P, Aho K, van de Putte LB (1997) HLA-DRB1 in eight Finnish monozygotic twin pairs concordant for rheumatoid arthritis. Tissue Antigens 49:277–279
Takeuchi F, Nakano K, Matsuta K et al. (1996) Positive and negative association of HLA-DR genotypes with Japanese rheumatoid arthritis. Clin Exp Rheumatol 14:17–22
Albani S, Tuckwell JE, Esparza L, Carson DA, Roudier J (1992) The susceptibility sequence to rheumatoid arthritis is a cross-reactive B cell epitope shared by the Escherichia coli heat shock protein dnaJ and the histocompatibility leukocyte antigen DRB10401 molecule. J Clin Invest 89:327–331
Uphoff TS, Welch RA (1990) Nucleotide sequencing of the Proteus mirabilis calcium independent hemolysin genes (hpmA and hpmB) reveals sequence similarity with the Serratia marcescens hemolysin genes (shlA and shlB). J Bacteriol 172:1206–1216
Poole K, Schiebel E, and Braun V (1988) Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol 170:3177–3188
Ebringer A, Cunningham P, Ahmadi K, Wrigglesworth J, Hosseini R, Wilson C (1992) Sequence similarity between HLA-DR1 and DR4 subtypes associated with rheumatoid arthritis and Proteus/Serratia membrane haemolysins. Ann Rheum Dis 51:1245–1246
Takeuchi F, Kosuge E, Matsuta K et al. (1990) Antibody to a specific HLA-DR beta 1 sequence in Japanese patients with rheumatoid arthritis. Arthritis Rheum 33:1867–1868
Wilson C, Ebringer A, Ahmadi K et al. (1995) Shared amino acid sequences between major histocompatibility complex class II glycoproteins, type XI collagen and Proteus mirabilis in rheumatoid arthritis. Ann Rheum Dis 54:216–220
Aho K, Koskenvuo M, Tuominen J, Kaprio J (1986). Occurrence of rheumatoid arthritis in a nationwide series of twins. J Rheumatol 13:899–902
Silman AJ, MacGregor AJ, Thomson W et al. (1993) Twin concordance rates for rheumatoid arthritis: results from a nation-wide study. Br J Rheumatol 32:903–907
Ebringer A, Ptaszynska T, Corbett M et al. (1985) Antibodies to Proteus in rheumatoid arthritis. Lancet ii: 305–307
Rogers P, Hassan J, Bresnihan B, Feighery C, Whelan A (1988) Antibodies to Proteus in rheumatoid arthritis. Br J Rheumatol 27 (Suppl 2): 90–94
Deighton CM, Gray JW, Bint AJ, Walker DJ (1992) Anti-Proteus antibodies in rheumatoid arthritis same-sexed sibships. Br J Rheumatol 31:241–245
Senior BW, McBride PD, Morley KD, Kerr MA (1995) The detection of raised level of IgM to Proteus mirabilis in sera from patients with rheumatoid arthritis. J Med Microbiol 43:176–184
Rashid T, Darlington G, Kjeldsen-Kragh J, Forre O, Collado A, Ebringer A (1999) Proteus IgG antibodies and C-reactive protein in English, Norwegian and Spanish patients with rheumatoid arthritis. Clin Rheumatol 18:190–195
Ebringer A, Rashid T, Wilson C (2003) Rheumatoid arthritis: proposal for the use of anti-microbial therapy in early cases. Scand J Rheumatol 32:2–11
Arnett FC, Edworthy SM, Bloch DA et al. (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Merrifield RB. Solid-phase peptide synthesis (1969) Adv Enzymol Relat Areas Mol Biol 32:221–296
Ebringer A, Cox NL, Abuljadayel I et al. (1988) Klebsiella antibodies in ankylosing spondylitis and Proteus antibodies in rheumatoid arthritis. Br J Rheumatol 27 (Suppl 2): 72–85
Deighton CM, Gray J, Bint AJ, Walker DJ (1992) Specificity of the Proteus antibody response in rheumatoid arthritis. Ann Rheum Dis 51:1206–1207
Kjeldsen-Kragh J, Rashid T, Dybward A et al. (1995) Decrease in anti-Proteus mirabilis but not anti-Escherichia coli antibody levels in rheumatoid arthritis patients treated with fasting and a one year vegetarian diet. Ann Rheum Dis 54:221–224
Fielder M, Tiwana H, Youinou P et al. (1995) The specificity of the anti-Proteus antibody response in tissue-typed rheumatoid arthritis (RA) patients from Brest. Rheumatol Int 15:79–82
Subair H, Tiwana H, Fielder M et al. (1995) Elevation in anti-Proteus antibodies in patients with rheumatoid arthritis from Bermuda and England. J Rheumatol 22:1825–1828
Tiwana H, Wilson C, Cunningham P, Binder A and Ebringer A (1996) Antibodies to four gram-negative bacteria in rheumatoid arthritis which share sequences with the rheumatoid arthritis susceptibility motif. Br J Rheumatol 35:592–594
Tani Y, Tiwana H, Hukuda S et al. (1997) Antibodies to Klebsiella, Proteus, and HLA-B27 peptides in Japanese patients with ankylosing spondylitis and rheumatoid arthritis. J Rheumatol 24:109–14
Murphy EA, Mowat L and Sturrock RD (1991) Antibodies to Proteus in rheumatoid arthritis. Br J Rheumatol 30:390
Dybwad A, Forre O, and Sioud M (1996) Increased serum and synovial antibodies to immunoselected peptides in patients with rheumatoid arthritis. Ann Rheum Dis 55:437–441
Wilson C, Rashid T, Tiwana H et al. (2003) Cytotoxicity responses to peptide antigens in rheumatoid arthritis and ankylosing spondylitis. J Rheumatol 30:972–978
Swihart KG, Welch RA (1990) The HpmA hemolysin is more common than HlyA among Proteus isolates. Infect Immun 58:1853–1860
Aknowledgements
We thank the Trustees of the Middlesex Hospital and the Arthritis Research Campaign for their support (Grant E0514).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rashid, T., Leirisalo-Repo, M., Tani, Y. et al. Antibacterial and antipeptide antibodies in Japanese and Finnish patients with rheumatoid arthritis. Clin Rheumatol 23, 134–141 (2004). https://doi.org/10.1007/s10067-003-0847-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-003-0847-6