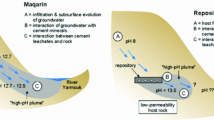
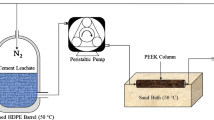
Abstract
Sulfate rocks are considered as problematic rocks due to their high solubility and deformation potential, when present in hydraulic structure’s foundation. In this paper, the effect of mineral composition on the dissolution rate constant (Kc) of these rocks has been studied. For this purpose, sulfate rock blocks were collected from the sulfate rock layer outcrops of Gachsaran Formation at the three under construction reservoir dam sites in Iran, and by preparing cylindrical core samples, dissolution simulation experiments were performed. For the purposes of the pressure (P) phase, a special pressure vessel was designed and manufactured to accommodate the simulation of dissolution in the static to water circulation at the desired pressures. The experiments were performed at pressures of 1 to 7.5 atmospheres. The water flow velocities (Vw) were set to a range between 0 and 0.008 m/s. The pH of the solution was maintained between 6.5 and 7 and a temperature of 25 °C. The results of this study confirmed that, firstly, there is a direct relationship between pressure and Kc. Secondly, as the water flow increases, the amount of dissolution and the Kc increase. Thirdly, sulfate rocks with different mineral composition experience different dissolution behaviors. One important outcome of this study is that, despite changes in P, Vw, and mineral composition the solubility of sulfate rocks at 25 °C in ionized water is about 1.9 g/l. Furthermore, all of considered factors affect only on the time of reaching the dissolution electrolyte to saturation or equilibrium condition.













Similar content being viewed by others
Data availability
All research data are presented in the tables of the article.
Code availability
No specific software program was used in this study that required specific code.
References
Adekola AF, Olosho AI, Baba AA, Adebayo SA (2018) Dissolution kinetics studies of Nigerian gypsum ore in hydrochloric acid. J Chem Technol 53(5):845–855
Aljubouri ZA, Al-Kawaz HA (2007) Dissolution Rate of Gypsum Under Different Environments. Iraqi J of Earth Sci 7(2):11–18
Allen RD, Kramer H (1953) Occurrence of bassanite in two desert basins in southeastern California. Am Mineral 38:1266–1268
Apokodje EG (1984) The occurrence of bassanite in some Australian arid-zone soils. Chem Geol 47:361–364. https://doi.org/10.1016/0009-2541(84)90135-9
ASTM C 471–87 (1987) Chemical Analysis of Gypsum and Gypsum Products
ASTM C 472–99 (2014) Standard Test Method for Physical Testing of Gypsum, Gypsum Plasters and Gypsum Concrete
ASTM D 2216–99 (1999) Standard Test Method for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass
ASTM D 4543 (2001) Standard practice for preparing rock core specimens and determining dimensional and shape tolerances Annual Book of ASTM Standards 04-02
ASTM D 4643 (2017) Standard Test Method for Determination of Water Content of Soil and Rock by Microwave Oven Heating Annual Book of ASTM Standards 04:08
Azimi G, Papangelakis VG, Dutrizac JE (2007) Modelling of calcium sulphate solubility in concentrated multi-component sulphate solutions. Fluid Phase Equilib 260(2):300–315. https://doi.org/10.1016/j.fluid.2007.07.069
Blount CW, Dickson FW (1973) Gypsum-anhydrite equilibria in systems CaSO4–H2O and CaSO4 –NaCl–H2O. Am Mineral 58:323–331
Bock E (1961) On the solubility of anhydrous calcium sulphate and of gypsum in concentrated solutions of sodium chloride at 25, 30, 40, and 50º C. Can J Chem 39(9):1746–1751
Cigna A (1985) Some remarks on phase equilibria of evaporates and other karstifiable rocks. Le Grotte D’ltalia 4(22):201–208
Colombani J, Bert J (2007) Holographic interferometry study of the dissolution and diffusion of gypsum in water. Geochim Cosmochim Acta 71(8):1913–1920. https://doi.org/10.1016/j.gca.2007.01.012
Cooper AH, Waltham AC (1999) Subsidence Caused by Gypsum Dissolution at Ripon, North Yorkshire. Q J Eng Geol Hydroge 32:305–310. https://doi.org/10.1144/GSL.QJEG.1999.032.P4.01
Day RW (2000) Geotechnical Engineer’s Portable Handbook. McGraw-Hill, New York, USA
Dickson FW, Blount CW, Tunell G (1963) Use of hydrothermal solution equipment to determine the solubility of anhydrite in water from 100° C to 275° C and from 1 bar to 1000 bars pressure. Am J Sci 261:61–78. https://doi.org/10.2475/ajs.261.1.61
Fengxiang C, Mingjiang W (1983) Investigation of the Engineering Properties of a Dam Foundation Containing Gypsum Seams. Rock Mech Rock Eng 16:275–280. https://doi.org/10.1007/BF01042361
Ford DC, Williams PK (2007) Hydrogeology and Geomorphology John Wiley, Chichester 562. https://doi.org/10.1002/9781118684986
Hong D, Fan M, Yu L (2018) An experimental study simulating the dissolution of gypsum rock. Energ Explor Exploit 36(2):014459871775192. https://doi.org/10.1177/0144598717751927
IS 1288 (1982) Methods of test for mineral gypsum. Bureau of Indian standards
James AN (1992) Soluble materials in civil engineering. Ellis Horwood, Chichester
James AN, Kirkpatrick IM (1980) Design of Foundations of Dams Containing Soluble Rock, s and Soils. Q J Eng Geol 13:189–198. https://doi.org/10.1144/GSL.QJEG.1980.013.03.05
James AN, Lupton ARR (1978) Gypsum and Anhydrite in Foundations of Hydraulic Structures. Geotechnique 28(3):249–272. https://doi.org/10.1680/geot.1978.28.3.249
Johnson KS (2005) Subsidence Hazards due to Evaporite Dissolution in the United States. Environ Geol 48:395–409. https://doi.org/10.1007/s00254-005-1283-5
Johnson KS (2008) Gypsum-Karst Problems in Constructing Dams in the USA. Environ Geol 53:945–950. https://doi.org/10.1144/10.1007/s00254-007-0720-z
Klimchouk AB (1996) The Dissolution and Conversion of Gypsum and Anhydrite. Int J Speleol 25(3–4):21–36. https://doi.org/10.5038/1827-806X.25.3.2
Korzhinsky DS (1953) Essays on Metasomatic Processes in Main Problems of the Geology of Magmatic Ore Deposits. Akad Nauk SSSR, Moscow. 47332–47553 (in Russian)
Lebedev AL, Lekhov A (1990) Dissolution kinetics of natural-gypsum in water at 5–25 °C. Geochem Int 27:85–94
Li J, Duan Z (2011) A thermodynamic model for the prediction of phase equilibria and speciation in the H2O-CO2-NaCl-CaCO3-CaSO4 system from 0 to 25o C, 1 to 1000 bar with NaCl concentrations up to halite saturation. GEOCHIM COSMOCHIM AC 75:4351–4376. https://doi.org/10.1016/j.gca.2011.05.019
Liang W, Xu S, Dusseault MB (2008) Dissolution and Seepage Coupling Effect on Transport and Mechanical Properties of Glauberite Salt Rock. Transp Porous 74:185–199. https://doi.org/10.1007/s11242-007-9190-8
Liley PE, Touloukian YS, Gambil WR (1963) Physical and chemical data. In: Perry.H. (Ed.) Chemical Engineer Handbook. 4th ed. McGraw-Hill Book Co
Liu Q, Lu Y, Zhang F (2013) Laboratory simulation experiment on dissolution of limestone under hydrodynamic pressure. Carbonate Evaporite 28:3–11. https://doi.org/10.1007/s13146-013-0159-0
Manikhin VI (1966) On the question of solubility of calcium sulphate under high pressures. Geokhimicheskie Materialy 13:193–196 (in Russian)
Mohammadi SD, Rahimi MR, Taleb Beydokhti A (2020) Saturation Procedures of Soluble Rocks (Emphasizing on Gypsum-Anhydrite Rocks). JIRAEG 13(3):97–111. http://www.jiraeg.ir/article_113119.html
Monnin C (1990) The influence of pressure on the activity-coefficients of the solutes and on the solubility of minerals in the system Na-Ca- Cl-SO4-H2O to 200° C and 1 kbar, and to high NaCl concentration. Geochim Cosmochim AC 54:3265–3282. https://doi.org/10.1016/0016-7037(90)90284-R
Nouri Sartangi M, Uromeihy A, Zarei K (2013) Evaluation of Geological Engineering Characteristics of Gachsaran Formation for Water Leakage (Case Study: Khorsan III Dam Reservoir). Geosci Sci Q J 105:131–142 ((in Persian))
Pecherkin AI (1986) Geodinamics of sulphate karst. Irkutsk University Publ (in Russian)
Peckmann J, Goedert JL, Heinrichs T, Hoefs J, Reitner J (2003) The late eocene ‘Whiskey Creek’ methane-seep deposit (western Washington State)-part II: petrology, stable isotopes, and biogeochemistry. Facies 48:241–254. https://doi.org/10.1007/BF02667542
Rahimi MR (2020) The study of the engineering geological properties of Gachsaran formation's gypsum and anhydrite rocks in some of the large reservoir dam sites in Iran, with emphasis on the dissolution behavior of rocks. Dissertation, Bu Ali Sina University, Iran (In Persian)
Rahimi MR, Mohammadi SD, Beydokhti AT (2020) Effects of Mineral Composition and Texture on Durability of Sulfate Rocks in Gachsaran Formation. Iran Geotech Geol Eng 38:2619–2637. https://doi.org/10.1007/s10706-019-01173-9
Rauh F, Spaun G, Thuro K (2006) Assessment of the swelling potential of anhydrite in tunneling projects. In: Pre-Congress Proceedings 10th IAEG Congress, paper no. 473. Nottingham, England
Razouki SS, El-Janabi OA (1999) Decrease in the CBR of Gypsiferous Soil Due to Long-Term Soaking. Q J Eng Geol Hydroge 32:87–89. https://doi.org/10.1144/GSL.QJEG.1999.032.P1.07
Razouki SS, Kuttah DK (2004) Distress of Light Structures and PMeanments over Swelling Gypsiferous Soils. Proceeding of International Conference on Geotechnical Engineering, 3–6 October. Sharjah, UAE
Razouki SS, Kuttah DK, Al-Damluji OA, Nashat LH (2006) Strength erosion of fine grained gypsiferous soil during soaking. Arab J Sci Eng 32(1):147–152
Salih NB (2013) Stability of dams constructed on problematic substrates. PhD Thesis. London, UK: Brunel University
Shafiei A, Dusseault MN, Baghdar-dokht Z (2008) Geotechnical Properties of Soluble Rocks from a Dam Site in Iran, The 2nd US Rock Mechanics Symposium and Us-Canada Rock Mechanics Symposium San Francisco
Shukla J, Mohandas VP, Kumar A (2008) Effect of pH on the Solubility of CaSO4·2H2O in Aqueous NaCl Solutions and Physicochemical Solution Properties at 35 °C. J Chem Eng Data 53(12):2797–2800. https://doi.org/10.1021/je800465f
Sonnenfeld P (1984) Brines and evaporates. Academic Press, London
Subhi Aziz A (1979) Creep properties of evaporite rocks with particular reference to gypsum. Dissertation, University of Sheffield, UK
Torabi-Kaveh M, Heidari M, Behzad HM, Mohammad M (2011) Effect of Dissolved Sodium Chloride Content ln Water on The Dissolution of Gypseous Rock (Case Study; Chamshir Dam Reservoir, SW Iran). Aust J Basic Appl Sci 5(9):1418–1424
Ulker R, Gumusoglu MC (1982) The Investigation of the Effect of Gypsum on Foundation Design. Bull Int Assoc Eng Geol 25:99–105. https://doi.org/10.1007/BF02603199
Wang W, Zeng D, Chen Q, Yin X (2013) Experimental determination and modeling of gypsum and insoluble anhydrite solubility in the system CaSO4–H2SO4–H2O. Chem Eng Sci 101:120–129. https://doi.org/10.1016/j.ces.2013.06.023
Warren JK (2006) Evaporites: sediments, resources and hydrocarbons. Springer, Berlin. https://doi.org/10.1007/3-540-32344-9
White BW (1977) Role of solution kinetics in the development of karst aquifers. Int Assoc Hydrogeol Mem 12:503–517
Yilmaz I (2001) Gypsum/anhydrite: some engineering problems. Bull Eng Geol Environ 59:227–230. https://doi.org/10.1007/s100640000071
Zheng HF, Duan TY, Liu Y, Sun Q (2009) Abrupt solubility of gypsum in water at high pressure and ambient temperature and its implication. Acta Petrol Sin 25(5):1288–1290
Funding
This work was supported by the president research office of Bu-Ali Sina University, Iran, (grant numbers 4.95, 2016).
Author information
Authors and Affiliations
Contributions
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Corresponding authors
Ethics declarations
Ethics approval
The research funding is referred to in “Funding” paragraph, and no living organisms are used in the experiments.
Consent to participate
Participated in this research with full consent.
Consent for publication
All three authors are agree with the paper content, and in their administrative affiliation, they have taken the necessary steps to publish the paper. All authors of this article express their consent to its publication.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahimi, M.R., Mohammadi, S.D. & Beydokhti, A.T. Correlation between the mineral composition and dissolution rate constant of sulfate rocks at different pressures and water flow velocities. Bull Eng Geol Environ 81, 371 (2022). https://doi.org/10.1007/s10064-022-02876-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10064-022-02876-9