Abstract
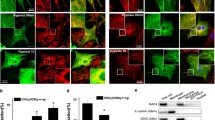
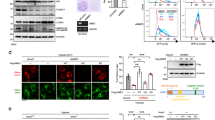
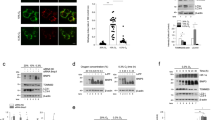
Hypoxia-induced microtubule disruption and mitochondrial permeability transition (mPT) are crucial events leading to fatal cell damage and recent studies showed that microtubules (MTs) are involved in the modulation of mitochondrial function. Dynein light chain Tctex-type 1 (DYNLT1) is thought to be associated with MTs and mitochondria. Previously we demonstrated that DYNLT1 knockdown aggravates hypoxia-induced mitochondrial permeabilization, which indicates a role of DYNLT1 in hypoxic cytoprotection. But the underlying regulatory mechanism of DYNLT1 remains illusive. Here we aimed to investigate the phosphorylation alteration of DYNLT1 at serine 82 (S82) in hypoxia (1% O2). We therefore constructed recombinant adenoviruses to generate S82E and S82A mutants, used to transfect H9c2 and HeLa cell lines. Development of hypoxia-induced mPT (MMP examining, Cyt c release and mPT pore opening assay), hypoxic energy metabolism (cellular viability and ATP quantification), and stability of MTs were examined. Our results showed that phosph-S82 (S82-P) expression was increased in early hypoxia; S82E mutation (phosphomimic) aggravated mitochondrial damage, elevated the free tubulin in cytoplasm and decreased the cellular viability; S82A mutation (dephosphomimic) seemed to diminish the hypoxia-induced injury. These data suggest that DYNLT1 phosphorylation at S82 is involved in MTs and mitochondria regulation, and their interaction and cooperation contribute to the cellular hypoxic tolerance. Thus, we provide new insights into a DYNLT1 mechanism in stabilizing MTs and mitochondria, and propose a potential therapeutic target for hypoxia cytoprotective studies.
Similar content being viewed by others
References
Al Rahim, M., Thatipamula, S., and Hossain, M.A. (2013). Critical role of neuronal pentraxin 1 in mitochondria-mediated hypoxicischemic neuronal injury. Neurobiol. Dis. 50, 59–68.
Belmont, L.D., and Mitchison, T.J. (1996). Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell 84, 623–631.
Brito, D.A., and Rieder, C.L. (2009). The ability to survive mitosis in the presence of microtubule poisons differs significantly between human nontransformed (RPE-1) and cancer (U2OS, HeLa) cells. Cell Motil. Cytoskel. 66, 437–447.
Cassimeris, L. (1999). Accessory protein regulation of microtubule dynamics throughout the cell cycle. Curr. Opin. Cell Biol. 11, 134–141.
Chuang, J.Z., Milner, T.A., and Sung, C.H. (2001). Subunit heterogeneity of cytoplasmic dynein: differential expression of 14 kDa dynein light chains in rat hippocampus. J. Neurosci. 21, 5501–5512.
Chuang, J.Z., Yeh, T.Y., Bollati, F., Conde, C., Canavosio, F., Caceres, A., and Sung, C.H. (2005). The dynein light chain Tctex-1 has a dynein-independent role in actin remodeling during neurite outgrowth. Dev. Cell 9, 75–86.
Fang, Y.D., Xu, X., Dang, Y.M., Zhang, Y.M., Zhang, J.P., Hu, J.Y., Zhang, Q., Dai, X., Teng, M., Zhang, D.X., et al. (2011). MAP4 mechanism that stabilizes mitochondrial permeability transition in hypoxia: microtubule enhancement and DYNLT1 interaction with VDAC1. PLoS One 6, e28052.
Feng, P., Liang, C., Shin, Y.C., Xiaofei, E., Zhang, W., Gravel, R., Wu, T.T., Sun, R., Usherwood, E., and Jung, J.U. (2007). A novel inhibitory mechanism of mitochondrion-dependent apoptosis by a herpesviral protein. PLoS Pathog. 3, e174.
Frezza, C., Zheng, L., Tennant, D.A., Papkovsky, D.B., Hedley, B.A., Kalna, G., Watson, D.G., and Gottlieb, E. (2011). Metabolic profiling of hypoxic cells revealed a catabolic signature required for cell survival. PLoS One 6, e24411.
Hein, S., Scheffold, T., and Schaper, J. (1995). Ischemia induces early changes to cytoskeletal and contractile proteins in diseased human myocardium. J. Thorac. Cardiovasc. Surg. 110, 89–98.
Hu, J.Y., Chu, Z.G., Han, J., Dang, Y.M., Yan, H., Zhang, Q., Liang, G.P., and Huang, Y.S. (2010). The p38/MAPK pathway regulates microtubule polymerization through phosphorylation of MAP4 and Op18 in hypoxic cells. Cell Mol. Life Sci. 67, 321–333.
Huang, Y.S., Yang, Z.C., Yan, B.G., Yang, J.M., Chen, F.M., Crowther, R.S., and Li, A. (1999). Pathogenesis of early cardiac myocyte damage after severe burns. J. Trauma 46, 428–432.
Huang, Y., Li, Z., and Yang, Z. (2003). Roles of ischemia and hypoxia and the molecular pathogenesis of post-burn cardiac shock. Burns 29, 828–833.
Kinnally, K.W., Peixoto, P.M., Ryu, S.Y., and Dejean, L.M. (2011a). Is mPTP the gatekeeper for necrosis, apoptosis, or both? Bba-Mol. Cell Res. 1813, 616–622.
Kinnally, K.W., Peixoto, P.M., Ryu, S.Y., and Dejean, L.M. (2011b). Is mPTP the gatekeeper for necrosis, apoptosis, or both? Biochim. Biophys. Acta 1813, 616–622.
Liang, B., Kong, D., Liu, Y., Liang, N., He, M., Ma, S., and Liu, X. (2012). Autophagy inhibition plays the synergetic killing roles with radiation in the multi-drug resistant SKVCR ovarian cancer cells. Radiat. Oncol. 7, 213.
Loos, B., Genade, S., Ellis, B., Lochner, A., and Engelbrecht, A.M. (2011). At the core of survival: autophagy delays the onset of both apoptotic and necrotic cell death in a model of ischemic cell injury. Exp. Cell Res. 317, 1437–1453.
Makokha, M., Hare, M., Li, M., Hays, T., and Barbar, E. (2002). Interactions of cytoplasmic dynein light chains Tctex-1 and LC8 with the intermediate chain IC74. Biochemistry 41, 4302–4311.
Maldonado, E.N., Patnaik, J., Mullins, M.R., and Lemasters, J.J. (2010). Free tubulin modulates mitochondrial membrane potential in cancer cells. Cancer Res. 70, 10192–10201.
McCommis, K.S., and Baines, C.P. (2012). The role of VDAC in cell death: friend or foe? Biochim. Biophys. Acta 1818, 1444–1450.
Mok, Y.K., Lo, K.W., and Zhang, M. (2001). Structure of Tctex-1 and its interaction with cytoplasmic dynein intermediate chain. J. Biol. Chem. 276, 14067–14074.
Nagano, F., Orita, S., Sasaki, T., Naito, A., Sakaguchi, G., Maeda, M., Watanabe, T., Kominami, E., Uchiyama, Y., and Takai, Y. (1998). Interaction of Doc2 with tctex-1, a light chain of cytoplasmic dynein. Implication in dynein-dependent vesicle transport. J. Biol. Chem. 273, 30065–30068.
Palmer, K.J., MacCarthy-Morrogh, L., Smyllie, N., and Stephens, D.J. (2011). A role for Tctex-1 (DYNLT1) in controlling primary cilium length. Eur. J. Cell Biol 90, 865–871.
Petronilli, V., Miotto, G., Canton, M., Brini, M., Colonna, R., Bernardi, P., and Di Lisa, F. (1999). Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 76, 725–734.
Petronilli, V., Penzo, D., Scorrano, L., Bernardi, P., and Di Lisa, F. (2001). The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J. Biol. Chem. 276, 12030–12034.
Putnam, A.J., Cunningham, J.J., Dennis, R.G., Linderman, J.J., and Mooney, D.J. (1998). Microtubule assembly is regulated by externally applied strain in cultured smooth muscle cells. J. Cell Sci. 111, 3379–3387.
Rogers, S.L., and Gelfand, V.I. (2000). Membrane trafficking, organelle transport, and the cytoskeleton. Curr. Opin. Cell Biol. 12, 57–62.
Rostovtseva, T.K., and Bezrukov, S.M. (2012). VDAC inhibition by tubulin and its physiological implications. Biochim. Biophys. Acta 1818, 1526–1535.
Rusanen, H., Majamaa, K., and Hassinen, I.E. (2000). Increased activities of antioxidant enzymes and decreased ATP concentration in cultured myoblasts with the 3243A—>G mutation in mitochondrial DNA. Biochim. Biophys. Acta 1500, 10–16.
Sato, H., Hori, M., Kitakaze, M., Iwai, K., Takashima, S., Kurihara, H., Inoue, M., and Kamada, T. (1993). Reperfusion after brief ischemia disrupts the microtubule network in canine hearts. Circ. Res. 72, 361–375.
Scharstuhl, A., Mutsaers, H.A., Pennings, S.W., Russel, F.G., and Wagener, F.A. (2009). Involvement of VDAC, Bax and ceramides in the efflux of AIF from mitochondria during curcumininduced apoptosis. PLoS One 4, e6688.
Schwarzer, C., Barnikol-Watanabe, S., Thinnes, F.P., and Hilschmann, N. (2002). Voltage-dependent anion-selective channel (VDAC) interacts with the dynein light chain Tctex 1 and the heat-shock protein PBP74. Int. J. Biochem. Cell Biol. 34, 1059–1070.
Seko, Y., Takahashi, M., Tobe, K., Kadowaki, T., and Yazaki, Y. (1997). Hypoxia and hypoxia/reoxygenation activate p65(PAK), p38mi-togen-activated protein kinase (MAPK), and stressactivated protein kinase (SAPK) in cultured rat cardiac myocytes. Biochem. Biophys. Res. Commun. 239, 840–844.
Sheldon, K.L., Maldonado, E.N., Lemasters, J.J., Rostovtseva, T.K., and Bezrukov, S.M. (2011). Phosphorylation of voltage-dependent anion channel by serine/threonine kinases governs its interaction with tubulin. PLoS One 6, e25539.
Song, Y., Benison, G., Nyarko, A., Hays, T.S., and Barbar, E. (2007). Potential role for phosphorylation in differential regulation of the assembly of dynein light chains. J. Biol. Chem. 282, 17272–17279.
Song, H.P., Zhang, L., Dang, Y.M., Yan, H., Chu, Z.G., and Huang, Y.S. (2010). The phosphatidylinositol 3-kinase-Akt pathway protects cardiomyocytes from ischaemic and hypoxic apoptosis via mitochondrial function. Clin. Exp. Pharmacol. Physiol. 37, 598–604.
Stavrovskaya, I.G., and Kristal, B.S. (2005). The powerhouse takes control of the cell: is the mitochondrial permeability transition a viable therapeutic target against neuronal dysfunction and death? Free Radic. Biol. Med. 38, 687–697.
Takeuchi, A., Mishina, Y., Miyaishi, O., Kojima, E., Hasegawa, T., and Isobe, K. (2003). Heterozygosity with respect to Zfp148 causes complete loss of fetal germ cells during mouse embryogenesis. Nat. Genet. 33, 172–176.
Teng, M., Dang, Y.M., Zhang, J.P., Zhang, Q., Fang, Y.D., Ren, J., and Huang, Y.S. (2010). Microtubular stability affects cardiomyocyte glycolysis by HIF-1alpha expression and endonuclear aggregation during early stages of hypoxia. Am. J. Physiol. Heart Circ. Physiol. 298, H1919–1931.
Tong, D.L., Zhang, D.X., Xiang, F., Teng, M., Jiang, X.P., Hou, J.M., Zhang, Q., and Huang, Y.S. (2012). Nicotinamide pretreatment protects cardiomyocytes against hypoxia-induced cell death by improving mitochondrial stress. Pharmacology 90, 11–18.
Vandroux, D., Schaeffer, C., Tissier, C., Lalande, A., Bes, S., Rochette, L., and Athias, P. (2004). Microtubule alteration is an early cellular reaction to the metabolic challenge in ischemic cardiomyocytes. Mol. Cell. Biochem. 258, 99–108.
Wan, X., O’Quinn, R.P., Pierce, H.L., Joglekar, A.P., Gall, W.E., DeLuca, J.G., Carroll, C.W., Liu, S.T., Yen, T.J., McEwen, B.F., et al. (2009). Protein architecture of the human kinetochore microtubule attachment site. Cell 137, 672–684.
Wang, G., Zhang, B.Q., Ruan, J., Luo, Z.H., Zhang, J.P., Xiao, R., Lei, Z.Y., Hu, J.Y., Chen, Y.S., and Huang, Y.S. (2012). Shaking stress aggravates burn-induced cardiovascular and renal disturbances in a rabbit model. Burns 39, 760–766.
Williams, J.C., Xie, H., and Hendrickson, W.A. (2005). Crystal structure of dynein light chain TcTex-1. J. Biol. Chem. 280, 21981–21986.
Yeh, T.Y., Peretti, D., Chuang, J.Z., Rodriguez-Boulan, E., and Sung, C.H. (2006). Regulatory dissociation of Tctex-1 light chain from dynein complex is essential for the apical delivery of rhodopsin. Traffic 7, 1495–1502.
Zang, Q., Maass, D.L., Tsai, S.J., and Horton, J.W. (2007). Cardiac mitochondrial damage and inflammation responses in sepsis. Surg. Infect. 8, 41–54.
Zhdanov, A.V., Dmitriev, R.I., and Papkovsky, D.B. (2011). Bafilomycin A1 activates respiration of neuronal cells via uncoupling associated with flickering depolarization of mitochondria. Cell. Mol. Life Sci. 68, 903–917.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Xu, X., Zhang, Q., Hu, Jy. et al. Phosphorylation of DYNLT1 at serine 82 regulates microtubule stability and mitochondrial permeabilization in hypoxia. Mol Cells 36, 322–332 (2013). https://doi.org/10.1007/s10059-013-0114-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-013-0114-x