Abstract
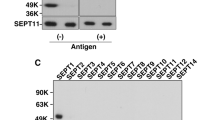
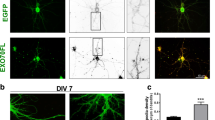
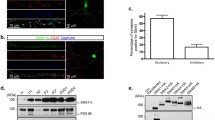
Septins, a conserved family of GTP-binding proteins with a conserved role in cytokinesis, are present in eukaryotes ranging from yeast to mammals. Septins are also highly expressed in neurons, which are post-mitotic cells. Septin6 (SEPT6) forms SEPT2/6/7 complexes in vivo. In this study, we produced a very specific SEPT6 antibody. Immunocytochemisty (ICC) of dissociated hippocampal cultures revealed that SEPT6 was highly expressed in neurons. Developmentally, the expression of SEPT6 was very low until stage 3 (axonal outgrowth). Significant expression of SEPT6 began at stage 4 (outgrowth of dendrites). At this stage, SEPT6 clusters were positioned at the branch points of developing dendrites. In maturing and mature neurons (stage 5), SEPT6 clusters were positioned at the base of filopodia and spines, and pre-synaptic boutons. Detergent extraction experiments also indicated that SEPT6 is not a post-synaptic density (PSD) protein. Throughout morphologic development of neurons, SEPT6 always formed tiny rings (external diameter, ∼0.5 μm), which appear to be clusters at low magnification. When a Sept6 RNAi vector was introduced at the early developmental stage (DIV 2), a significant reduction in dendritic length and branch number was evident. Taken together, our results indicate that SEPT6 begins to be expressed at the stage of dendritic outgrowth and regulates the cytoarchitecture.
Similar content being viewed by others
References
Bayer, S.A., and Altman, J. (1995). Neurogenesis and neuronal migration. In The Rat Nervous System, 2nd ed., G. Paxinos, ed. (San Diego, USA: Academic Press Inc.), pp. 1041–1078.
Bläser, S., Röseler, S., Rempp, H., Bartsch, I., Bauer, H., Lieber, M., Lessmann, E., Weingarten, L., Busse, A., Huber, M., et al. (2006). Human endothelial cell septins: SEPT11 is an interaction partner of SEPT5. J. Pathol. 210, 103–110.
Buckley, K., and Kelly, R.B. (1985). Identification of a transmembrane glycoprotein specific for secretory vesicles of neural and endocrine cells. J. Cell Biol. 100, 1284–1294.
Byers, B., and Goetsch, L. (1976). A highly ordered ring of membrane-associated filaments in budding yeast. J. Cell Biol. 69, 717–721.
Cao, L., Yu, W., Wu, Y., and Yu, L. (2009). The evolution, complex structures and function of septin proteins. Cell Mol. Life Sci. 66, 3309–3323.
Carlin, R.K., Grab, D.J., Cohen, R.S., and Siekevitz, P. (1980). Isolation and characterization of postsynpatic densities form various brain regions: enrichment of different types of postsynaptic densities. J. Cell Biol. 86, 831–843.
Caudron, F., and Barral, Y. (2009). Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell 16, 493–506.
Cohen, R.S., Chung, S.K., and Pfaff, D.W. (1985). Immunocytochemical localization of actin in dendritic spines of the cerebral cortex using colloidal gold as a probe. Cell. Mol. Neurobiol. 5, 271–284.
Collins, M.O., Husi, H., Yu, L., Brandon, J.M., Anderson, C.N., Blackstock, W.P., Choudhary, J.S., and Grant, S.G. (2005). Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 1(Suppl), 16–23.
Dotti, C.G., Sullivan, C.A., and Banker, G.A. (1988). The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 8, 1454–1468.
Douglas, L.M., Alvarez, F.J., McCreary, C., and Konopka, J.B. (2005). Septin function in yeast model systems and pathogenic fungi. Eukaryot. Cell 4, 1503–1512.
Fritschy, J.M., Harvey, R.J., and Schwarz, G. (2008). Gephyrin: where do we stand, where do we go? Trends Neurosci. 31, 257–264.
Fujishima, K., Kiyonari, H., Kurisu, J., Hirano, T., and Kengaku, M. (2007). Targeted disruption of Sept3, a heteromeric assembly partner of Sept5 and Sept7 in axons, has no effect on developing CNS neurons. J. Neurochem. 102, 77–92.
Garner, C.C., Waites, C.L., and Ziv, N.E. (2006). Synapse development: Still looking for the forest, still lost in the trees. Cell Tissue Res. 326, 249–262.
Gladfelter, A.S., Pringle, J.R., and Lew, D.J. (2001). The septin cortex at the yeast mother-bud neck. Curr. Opin. Microbiol. 4, 681–689.
Goslin, K., Assmussen, H., and Banker, G. (1998). Rat hippocampal neurons in low density culture. In Culturing Nerve Cells, 2nd ed., G. Banker and K. Goslin, eds. (Cambridge, MIT Press), pp. 339–370.
Hall, P.A., Jung, K., Hillan, K.J., and Russell, S.E. (2005). Expression profiling the human septin gene family. J. Pathol. 206, 269–278.
Hanai, N., Nagata, K., Kawajiri, A., Shiromizu, T., Saitoh, N., Hasegawa, Y., Murakami, S., and Inagaki, M. (2004). Biochemical and cell biological characterization of a mammalian septin, Sept11. FEBS Lett. 568, 83–88.
Harris, K.M., and Kater, S.B. (1994). Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu. Rev. Neurosci. 17, 341–371.
Hartwell, L.H. (1971). Genetic control of the cell division cycle in yeast. IV. Genes controlling bud emergence and cytokinesis. Exp. Cell Res. 69, 265–276.
Hartwell, L., Culotti, J., and Reid, B. (1970). Genetic control of the cell-division cycle in yeast. I. Detection of mutants. Proc. Natl. Acad. Sci. USA 66, 352–359.
Hartwell, L.H., Culotti, J., Pringle, J.R., and Reid, B.J. (1974). Genetic control of the cell division cycle in yeast. Science 183, 46–51.
Ihara, M., Kinoshita, A., Yamada, S., Tanaka, H., Tanigaki, A., Kitano, A., Goto, M., Okubo, K., Nishiyama, H., Ogawa, O., et al. (2005). Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev. Cell 8, 343–352.
Kinoshita, M. (2006). Diversity of septin scaffolds. Curr. Opin. Cell Biol. 18, 54–60.
Kinoshita, A., Noda, M., and Kinoshita, M. (2000). Differential localization of septins in the mouse brain. J. Comp. Neurol. 428, 223–239.
Kinoshita, M., Field, C.M., Coughlin, M.L., Straight, A.F., and Mitchison, T.J. (2002). Self- and actin-templated assembly of Mammalian septins. Dev. Cell 3, 791–802.
Kneussel, M., and Loebrich, S. (2007). Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biol. Cell 99, 297–309.
Kremer, B.E., Haystead, T., and Macara, I.G. (2005). Mammalian septins regulate microtubule stability through interaction with the microtubule-binding protein MAP4. Mol. Biol. Cell 16, 4648–4659.
Li, X., Serwanski, D.R., Miralles, C.P., Nagata, K., and De Blas, A.L. (2009). Septin 11 is present in GABAergic synapses and plays a functional role in the cytoarchitecture of neurons and GABAergic synaptic connectivity. J. Biol. Chem. 284, 17253–17265.
Longtine, M.S., and Bi, E. (2003). Regulation of septin organization and function in yeast. Trends Cell Biol. 13, 403–409.
Longtine, M.S., DeMarini, D.J., Valencik, M.L., Al-Awar, O.S., Fares, H., De Virgilio, C., and Pringle, J.R. (1996). The septins: roles in cytokinesis and other processes. Curr. Opin. Cell Biol. 8, 106–119.
Luscher, B., and Keller, C.A. (2004). Regulation of GABAA receptor trafficking, channel activity, and functional plasticity of inhibitory synapses. Pharmacol. Ther. 102, 195–221.
Matus, A., Ackermann, M., Pehling, G., Byers, H.R., and Fujiwara, K. (1982). High actin concentrations in brain dendritic spines and postsynaptic densities. Proc. Natl. Acad. Sci. USA 79, 7590–7594.
McMurray, M.A., and Thorner, J. (2009). Septins: molecular partitioning and the generation of cellular asymmetry. Cell Div. 4, 18.
Moons, I.S., Apperson, M.L., and Kennedy, M.B. (1994). The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc. Natl. Acad. Sci. USA 91, 3954–3958.
Moon, I.S., Cho, S.J., Jin, I., and Walikonis, R. (2007). A simple method for combined fluorescence in situ hybridization and immunocytochemistry. Mol. Cells 24, 76–82.
Murphy, J.A., Jensen, O.N., and Walikonis, R.S. (2006). BRAG1, a Sec7 domain-containing protein, is a component of the postsynaptic density of excitatory synapses. Brain Res. 1120, 35–45.
Oh, Y., and Bi, E. (2011). Septin structure and function in yeast and beyond. Trends Cell Biol. 21, 141–148.
Ono, R., Ihara, M., Nakajima, H., Ozaki, K., Kataoka-Fujiwara, Y., Taki, T., Nagata, K., Inagaki, M., Yoshida, N., Kitamura, T., et al. (2005). Disruption of Sept6, a fusion partner gene of MLL, does not affect ontogeny, leukemogenesis induced by MLL-SEPT6, or phenotype induced by the loss of Sept4. Mol. Cell. Biol. 25, 10965–10978.
Park, H.J., Park, H.W., Lee, S.J., Arevalo, J.C., Park, Y.S., Lee, S.P., Paik, K.S., Chao, M.V., and Chang, M.S. (2010). Ankyrin repeat-rich membrane spanning/Kidins220 protein interacts with mammalian Septin 5. Mol. Cells 30, 143–148.
Peng, X.R., Jia, Z., Zhang, Y., Ware, J., and Trimble, W.S. (2002). The septin CDCrel-1 is dispensable for normal development and neurotransmitter release. Mol. Cell. Biol. 22, 378–387.
Peng, J., Kim, M.J., Cheng, D., Duong, D.M., Gygi, S.P., and Sheng, M. (2004). Semiquantitative proteomic analysis of rat forebrain postsynaptic density fractions by mass spectrometry. J. Biol. Chem. 279, 21003–21011.
Scheiffele, P., Fan, J., Choih, J., Fetter, R., and Serafini, T. (2000). Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 101, 657–669.
Sekino, Y., Kojima, N., and Shirao, T. (2007). Role of actin cytoskeleton in dendritic spine morphogenesis. Neurochem. Int. 51, 92–104.
Sheffield, P.J., Oliver, C.J., Kremer, B.E., Sheng, S., Shao, Z., and Macara, I.G. (2003). Borg/septin interactions and the assembly of mammalian septin heterodimers, trimers, and filaments. J. Biol. Chem. 278, 3483–3488.
Silverman-Gavrila, R.V., and Silverman-Gavrila, L.B. (2008). Septins: new microtubule interacting partners. Scientific World Journal 8, 611–620.
Spiliotis, E.T., Kinoshita, M., and Nelson, W.J. (2005). A mitotic septin scaffold required for Mammalian chromosome congression and segregation. Science 307, 1781–1785.
Stan, A., Pielarski, K.N., Brigadski, T., Wittenmayer, N., Fedorchenko, O., Gohla, A., Lessmann, V., Dresbach, T., and Gottmann, K. (2010). Essential cooperation of N-cadherin and neuroligin-1 in the transsynaptic control of vesicle accumulation. Proc. Natl. Acad. Sci. USA 107, 11116–11121.
Suzuki, G., Harper, K.M., Hiramoto, T., Sawamura, T., Lee, M., Kang, G., Tanigaki, K., Buell, M., Geyer, M.A., Trimble, W.S., et al. (2009). Sept5 deficiency exerts pleiotropic influence on affective behaviors and cognitive functions in mice. Hum. Mol. Genet. 18, 1652–1660.
Tada, T., Simonetta, A., Batterton, M., Kinoshita, M., Edbauer, D., and Sheng, M. (2007). Role of Septin cytoskeleton in spine morphogenesis and dendrite development in neurons. Curr. Biol. 17, 1752–1758.
Xie, Y., Vessey, J.P., Konecna, A., Dahm, R., Macchi, P., and Kiebler, M.A. (2007). The GTP-binding protein Septin 7 is critical for dendrite branching and dendritic-spine morphology. Curr. Biol. 17, 1746–1751.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Cho, SJ., Lee, H., Dutta, S. et al. Septin 6 regulates the cytoarchitecture of neurons through localization at dendritic branch points and bases of protrusions. Mol Cells 32, 89–98 (2011). https://doi.org/10.1007/s10059-011-1048-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-011-1048-9