Abstract
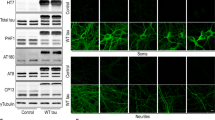
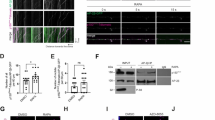
Lysophosphatidic acid (LPA) is a lipid growth factor that exerts diverse biological effects, including rapid neurite retraction and cell migration. Alterations in cell morphology, including neurite retraction, in neurodegenerative disorders such as Alzheimer’s disease involve hyperphosphorylation of the cytoskeletal protein tau. Since LPA has been shown to induce neurite retraction in various cultured neural cells and the detailed underlying molecular mechanisms have not yet been elucidated, we investigated whether LPA induced neurite retraction through taumediated signaling pathways in differentiated neuroblastoma cells. When Neuro2a cells differentiated with retinoic acid (RA) were exposed to LPA, cells exhibited neurite retraction in a time-dependent manner. The retraction of neurites was accompanied by the phosphorylation of tau. The LPA-induced neurite retraction and tau phosphorylation in differentiated Neuro2a cells were significantly abolished by the glycogen synthase kinase-3β (GSK-3β) inhibitor lithium chloride. Interestingly, the LPA-stimulated tau phosphorylation and neurite retraction were markedly prevented by the administration of H89, an inhibitor of both cyclic-AMP dependent protein kinase (PKA) and cyclic-AMP response element-binding protein (CREB). Transfection of the dominant-negative CREBs, K-CREB and A-CREB, failed to prevent LPA-induced tau phosphorylation and neurite retraction in differentiated Neuro2a cells. Taken together, these results suggest that GSK-3β and PKA, rather than CREB, play important roles in tau phosphorylation and neurite retraction in LPA-stimulated differentiated Neuro2a cells.
Similar content being viewed by others
References
Anliker, B., and Chun, J. (2004). Cell surface receptors in lysophospholipid signaling. Semin. Cell Dev. Biol. 15, 457–465.
Bhat, R.V., Shanley, J., Correll, M.., Fieles, W.E., Keith, R.A., Scott, C.W., and Lee, C.M. (2000). Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3beta in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 97, 11074–11079.
Boer, U., Eglins, J., Krause, D., Schnell, S., Schofl, C., and Knepel, W. (2007). Enhancement by lithium of cAMP-induced CRE/CREB-directed gene transcription conferred by TORC on the CREB basic leucine zipper domain. Biochem. J. 408, 69–77.
Cai, X., Li, M., Vrana, J., and Schaller, M.D. (2006). Glycogen synthase kinase — and extracellular signal-regulated kinasedependent phosphorylation of paxillin regulates cytoskeletal rearrangement. Mol. Cell. Biol. 26, 2857–2868.
Chomczynski, P., and Sacchi, N. (1987). Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162, 156–159.
Chun, J. (2005). Lysophospholipids in the nervous system. Prostaglandins Other Lipid Mediat. 77, 46–51.
Chun, J., Goetzl, E.J., Hla, T., Igarashi, Y., Lynch, K.R., Moolenaar, W., Pyne, S., and Tigyi, G. (2002). International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 54, 265–269.
Contos, J.J., Ishii, I., and Chun, J. (2000). Lysophosphatidic acid receptors. Mol. Pharmacol. 58, 1188–1196.
Evans, D.B., Rank, K.B., Bhattacharya, K., Thomsen, D.R., Gurney, M.E., and Sharma, S.K. (2000). Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J. Biol. Chem. 275, 24977–24983.
Fukushima, N. (2004). LPA in neural cell development. J. Cell Biochem. 92, 993–1003.
Fukushima, N., and Chun, J. (2001). The LPA receptors. Prostaglandins 64, 21–32.
Fukushima, N., Weiner, J.A., Kaushal, D., Contos, J.J., Rehen, S.K., Kingsbury, M.A., Kim, K.Y., and Chun, J. (2002). Lysophosphatidic acid influences the morphology and motility of young, postmitotic cortical neurons. Mol. Cell. Neurosci. 20, 271–282.
Ginty, D.D., Glowacka, D., Bader, D.S., Hidaka, H., and Wagner, J.A. (1991). Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J. Biol. Chem. 266, 17454–17458.
Goetzl, E.J., and An, S. (1999). A subfamily of G protein-coupled cellular receptors for lysophospholipids and lysosphingolipids. Adv. Exp. Med. Biol. 469, 259–264.
Greenberg, S.G., and Davies, P. (1990). A preparation of Alzheimer paired helical filaments that displays distinct tau proteins by polyacrylamide gel electrophoresis. Proc. Natl. Acad. Sci. USA 87, 5827–5831.
Grimes, C.A., and Jope, R.S. (2001). CREB DNA binding activity is inhibited by glycogen synthase kinase-3 beta and facilitated by lithium. J. Neurochem. 78, 1219–1232.
Hartigan, J.A., and Johnson, G.V. (1999). Transient increases in intracellular calcium result in prolonged site-selective increases in Tau phosphorylation through a glycogen synthase kinase 3beta-dependent pathway. J. Biol. Chem. 274, 21395–21401.
Hu, X., Chen, J., Wang, L., and Ivashkiv, L.B. (2007). Crosstalk among Jak-STAT, Toll-like receptor, and ITAM-dependent pathways in macrophage activation. J. Leukoc. Biol. 82, 237–243.
Jalink, K., Eichholtz, T., Postma, F.R., van Corven, E.J., and Moolenaar, W.H. (1993). Lysophosphatidic acid induces neuronal shape changes via a novel, receptor-mediated signaling pathway: similarity to thrombin action. Cell Growth Differ. 4, 247–255.
Klein, P.S., and Melton, D.A. (1996). A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 93, 8455–8459.
Kwon, Y.J., Sun, Y., Kim, N.H., and Huh, S.O. (2009). Phosphorylation of CREB, a cyclic AMP responsive element binding protein, contributes partially to lysophosphatidic acid-induced fibroblast cell proliferation. Biochem. Biophys. Res. Commun. 380, 655–659.
Lee, C.W., Nam, J.S., Park, Y.K., Choi, H.K., Lee, J.H., Kim, N.H., Cho, J., Song, D.K., Suh, H.W., Lee, J., et al. (2003). Lysophosphatidic acid stimulates CREB through mitogen- and stressactivated protein kinase-1. Biochem. Biophys. Res. Commun. 305, 455–461.
Lee, C.W., Rivera, R., Gardell, S., Dubin, A.E., and Chun, J. (2006). GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J. Biol. Chem. 281, 23589–23597.
Li, X., Lu, F., Tian, Q., Yang, Y., Wang, Q., and Wang, J.Z. (2006). Activation of glycogen synthase kinase-3 induces Alzheimer-like tau hyperphosphorylation in rat hippocampus slices in culture. J. Neural Transm. 113, 93–102.
Mandell, J.W., and Banker, G.A. (1996). Microtubule-associated proteins, phosphorylation gradients, and the establishment of neuronal polarity. Perspect. Dev. Neurobiol. 4, 125–135.
Mao, A.J., Bechberger, J., Lidington, D., Galipeau, J., Laird, D.W., and Naus, C.C. (2000). Neuronal differentiation and growth control of neuro-2a cells after retroviral gene delivery of connexin43. J. Biol. Chem. 275, 34407–34414.
Moolenaar, W.H. (1999). Bioactive lysophospholipids and their G protein-coupled receptors. Exp. Cell Res. 253, 230–238.
Nam, J.H., Shin, D.H., Min, J.E., Ye, S.K., Jeon, J.H., and Kim, S.J. (2010). Ca2+ signaling induced by sphingosine 1-phosphate and lysophosphatidic acid in mouse B cells. Mol. Cells 29, 85–91.
Saito, S. (1997). Effects of lysophosphatidic acid on primary cultured chick neurons. Neurosci. Lett. 229, 73–76.
Sanchez, S., Sayas, C.L., Lim, F., Diaz-Nido, J., Avila, J., and Wandosell, F. (2001). The inhibition of phosphatidylinositol-3-kinase induces neurite retraction and activates GSK3. J. Neurochem. 78, 468–481.
Sayas, C.L., Moreno-Flores, M.T., Avila, J., and Wandosell, F. (1999). The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 274, 37046–37052.
Sayas, C.L., Avila, J., and Wandosell, F. (2002a). Regulation of neuronal cytoskeleton by lysophosphatidic acid: role of GSK-3. Biochim. Biophys. Acta 1582, 144–153.
Sayas, C.L., Avila, J., and Wandosell, F. (2002b). Glycogen synthase kinase-3 is activated in neuronal cells by Galpha12 and Galpha13 by Rho-independent and Rho-dependent mechanisms. J. Neurosci. 22, 6863–6875.
Sayas, C.L., Ariaens, A., Ponsioen, B., and Moolenaar, W.H. (2006). GSK-3 is activated by the tyrosine kinase Pyk2 during LPA1-mediated neurite retraction. Mol. Biol. Cell 17, 1834–1844.
Stambolic, V., Ruel, L., and Woodgett, J.R. (1996). Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 6, 1664–1668.
Steiner, B., Mandelkow, E.M., Biernat, J., Gustke, N., Meyer, H.E., Schmidt, B., Mieskes, G., Soling, H.D., Drechsel, D., Kirschner, M.W., et al. (1990). Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 9, 3539–3544.
Tanaka, T., Iqbal, K., Trenkner, E., Liu, D.J., and Grundke-Iqbal, I. (1995). Abnormally phosphorylated tau in SY5Y human neuroblastoma cells. FEBS Lett. 360, 5–9.
Tatebayashi, Y., Planel, E., Chui, D.H., Sato, S., Miyasaka, T., Sahara, N., Murayama, M., Kikuchi, N., Yoshioka, K., Rivka, R., et al. (2006). c-jun N-terminal kinase hyperphos-phorylates R406W tau at the PHF-1 site during mitosis. FASEB J. 20, 762–764.
Thomson, S., Clayton, A.L., Hazzalin, C.A., Rose, S., Barratt, M.J., and Mahadevan, L.C. (1999). The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J. 18, 4779–4793.
Tigyi, G., and Miledi, R. (1992). Lysophosphatidates bound to serum albumin activate membrane currents in Xenopus oocytes and neurite retraction in PC12 pheochromocytoma cells. J. Biol. Chem. 267, 21360–21367.
Yanagida, K., Ishii, S., Hamano, F., Noguchi, K., and Shimizu, T. (2007). LPA4/p2y9/GPR23 mediates rho-dependent morphological changes in a rat neuronal cell line. J. Biol. Chem. 282, 5814–5824.
Ye, X., Ishii, I., Kingsbury, M.A., and Chun, J. (2002). Lysophosphatidic acid as a novel cell survival/apoptotic factor. Biochim. Biophys. Acta 1585, 108–113.
Zhong, S., Jansen, C., She, Q.B., Goto, H., Inagaki, M., Bode, A.M., Ma, W.Y., and Dong, Z. (2001). Ultraviolet B-induced phosphorylation of histone H3 at serine 28 is mediated by MSK1. J. Biol. Chem. 276, 33213–33219.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Sun, Y., Kim, NH., Yang, H. et al. Lysophosphatidic acid induces neurite retraction in differentiated neuroblastoma cells via GSK-3β activation. Mol Cells 31, 483–489 (2011). https://doi.org/10.1007/s10059-011-1036-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-011-1036-0