Abstract
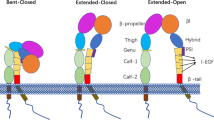
Integrin αXβ2 performs a significant role in leukocyte functions including phagocytosis and migration, and binds to a variety of ligands, including fibrinogen, iC3b, and ICAM-1. A particular domain of the α subunit of the integrin — the αX I-domain — is a ligand binding site, and the interaction of the αX I-domain and ICAM-1 on the endothelium is an important step in leukocyte extravasation. In order to elucidate the structural aspects of this interaction, we defined the moieties of the αX and ICAM-1 relevant to their interaction in this study. It was determined that the ICAM-1 binding sites of the αX I-domain were located in the α3α4, βDα5, and βFα7 loops at the top surface of the I-domain. The residues Q202, K242, K243, E298 and D299 on these loops were crucial for the recognition of ICAM-1. Among these residues, K242 and K243 on the βDα5 loop were found to be the most salient, thereby suggesting an ionic interaction between these proteins. Domain 3 of ICAM-1 was identified as a primary binding site for the αX I-domain. Two regions of domain 3 (D229QRLNPTV and E254DEGTQRL) perform critical functions in the binding of the αX I-domain. Especially, the residue E254DEG, is most important with regard to the αX I-domain.
Similar content being viewed by others
References
Arnaout, M.A. (2002). Integrin structure: new twists and turns in dynamic cell adhesion. Immunol. Rev. 186, 125–140.
Arnaout, M.A. Mahalingam, B., and Xiong, J.P. (2005). Integrin structure, allostery, and bidirectional signaling. Annu. Rev. Cell Dev. Biol. 21, 381–410.
Bilsland, C.A., Diamond, M.S., and Springer, T.A. (1994). The leukocyte integrin p150,95 (CD11c/CD18). as a receptor for iC3b. Activation by a heterologous β subunit and localization of a ligand recognition site to the I-domain. J. Immunol. 152, 4582–4589.
Bullard, D.C., Hu, X., Adams, J., Schoeb, T.R., and Barnum, S.R. (2007). p150/95 (CD11c/CD18). expression is required for the development of experimental auto immune encephalomyelitis. Immunol. Infec. Diseas. 170, 2001–2008.
Choi, J., Leyton, L., and Nham, S.-U. (2005). Characterization of αX I-domain binding to Thy-1. Biochem. Biophys. Res. Commun. 331, 557–561.
Diamond, M.S., Staunton, D.E., Marlin, S.D., and Springer, T.A. (1991). Binding of the integrin Mac-1(CD11b/CD18). to the third Ig-like domain of ICAM-1 (CD54). and its regulation by glycosylation. Cell 65, 961–971.
Frick, C., Odermatt, A., Zen, K., Mandell, K.J., Edens, H., Portmann, R., Mazzucchelli, R., Jaye, D.L., and Parkos, C.A. (2005). Interaction of ICAM-1 with β2-integrin CD11c/CD18: characterization of a peptide ligand that mimics a putative binding site on domain D4 of ICAM-1. Eur. J. Immunol. 35, 3610–3621.
Gang, J., Choi, J., Lee, J., and Nham, S.-U. (2007). Identification of critical residues for plasminogen binding by the αX I-domain. Mol. Cells 24, 240–246.
Harris, E.S., McIntyre, T.M., Prescott, S.M., and Zimmerman, G.A. (2000). The leukocyte integrins. J. Biol. Chem. 275, 23409–23412.
Ihanus, E., Uotila, L.M., Toivanen, A., Varis, M., and Gahmberg, C.G. (2007). Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: characterization of the binding sites on ICAM-4. Blood 109, 802–810.
Languino, L. R., Plescia, J., Duperray, A., Brian, A.A., Plow, E.F., Geltosky, J.E., and Altieri, D.C. (1993). Fibrinogen mediates leukocyte adhesion to vascular endothelium through an ICAM-1 dependent pathway. Cell 73, 1423–1434.
Lawson, C., and Wolf, S. (2009). ICAM-1 signaling in endothelial cells. Pharm. Report. 61, 22–32.
Lee, J.H., Choi, J., and Nham, S.-U. (2007). Critical residues of alpha X I-domain recognizing fibrinogen central domain. Biochem. Biophys. Res. Commun. 355, 1058–1063.
Loike, J.D., Sodeik, B., Cao, L., Leucona, S., Weitz, J.I., Detmers, P.A., Wright, S.D., and Silverstein, S.C. (1991). CD11c/CD18 on neutrophils recognizes a domain at the N terminus of the A alpha chain of fibrinogen. Proc. Natl. Acad. Sci. USA 88, 1044–1048.
Luo, B.-H., Carman, C.V., and Springer, T.A. (2007). Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647.
Metlay, J.P., Witmer-Pack, M.D., Agger, R., Crowley, M.T., Lawless, D., and Steinman, R.M. (1990). The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J. Exp. Med. 171, 1753–1771.
Meunier, L., Bohjanen, K., Voorhees, J.J., and Cooper, K.D. (1994). Retinoic acid upregulates human Langerhans cell antigen presentation and surface expression of HLA-DR and CD11c, a β2 integrin critically involved in T-cell activation. J. Invest. Dermatol. 103, 775–779.
Myones, B.L., Dalzell, J.G., Hogg, N., and Ross, G.D. (1988). Neutrophil and monocyte cell surface p150,95 has iC3b-receptor (CR4). activity resembling CR3. J. Clin. Invest. 82, 640–651.
Pettersen, E.F., Goddard, T.D., Huang, C.C., Couch, G.S., Greenblatt, D.M., Meng, E.C., and Ferrin, T.E. (2004). UCSF Chimeraa visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612.
Plow, E.F., Haas, T.A., Zhang, L., Loftus, J., and Smith, J.W. (2000). Ligand binding to integrin. J. Biol. Chem. 275, 21785–21788.
Sadhu, C., Ting, H.J., Lipsky, B., Hensley, K., Garcia-Martinez, L.F., Simon, S.I., and Staunton, D.E. (2007). CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J. Leuk. Biol. 81, 395–1403.
Schneider, P. (2000). Production of recombinant TRAIL and TRAIL receptor: Fc chimeric proteins. Methods Enzymol. 322, 325–345.
Shortman, K., and Liu, Y.J. (2002). Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2, 151–161.
Stacker, S.A., and Springer, T.A. (1991). Leukocyte integrin p150,95 (CD11c/CD18). functions as an adhesion molecule binding to a counter-receptor on stimulated endothelium. J. Immunol. 146, 648–655.
Stanley, P., and Hogg, N. (1998). The I-domain of integrin LFA-1 interacts with ICAM-1 domain 1 at residue Glu-34 but not Gln-73. J. Biol. Chem. 273, 3358–3362.
Staunton, D.E., Dustin, M.L., Erickson, H.P., and Springer, T.A. (1990). The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell 61, 243–254.
te Velde, A.A., Keizer, G.D., and Figdor, C.G. (1987). Differential function of LFA-1 family molecules (CD11 and CD18). in adhesion of human monocytes to melanoma and endothelial cells. Immunology 61, 261–267.
van Buul, J.D., Kanters, E., and Hordijk, P.L. (2007). Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler. Thromb. Vasc. Biol. 7, 1870–1876.
Vorup-Jensen, T., Ostermeier, C., Shimaoka, M., Hommel, U., and Springer, T.A. (2003). Structure and allosteric regulation of the αXβ2 integrin I-domain. Proc. Natl. Acad. Sci. USA 100, 1873–1878.
Vorup-Jensen, T., Carman, C.V., Shimaoka, M., Schuck, P., Svitel, J., and Springer T.A. (2005). Exposure of acidic residues as a danger signal for recognition of fibrinogen and other macromolecules by integrin. Proc. Natl. Acad. Sci. USA 102. 1614–1619.
Yang, Y., Jun, C.-D., Liu, J.-H., Zhang, R., Joachimiak, A., Springer, T.A., and Wang, J.-H. (2004). Structural basis for dimerization of ICAM-1 on the cell surface. Mol. Cell 14, 269–276.
Zang, Q., and Springer, T.A. (2001). Amino acid residues in the PSI-domain and cysteine-rich repeats of the integrin β2 subunit that restrain activation of the integrin αXβ2. J. Biol. Chem. 276, 6922–6929.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Choi, J., Choi, J. & Nham, SU. Characterization of the residues of αX I-domain and ICAM-1 mediating their interactions. Mol Cells 30, 227–234 (2010). https://doi.org/10.1007/s10059-010-0111-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0111-2