Abstract
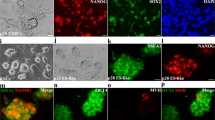
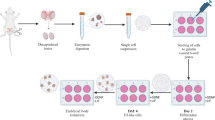
The aim of this study was to understand the mechanisms that allow mSSC lines to be established from SSCs. Small, multilayer clumps of SSCs formed during two to four weeks of in vitro culture and were then transferred to MEF feeders. Small, round, monolayer colonies containing cells destined to convert to mSSCs, designated as intermediate state SSCs (iSSCs), first appeared after two to three passages. During an additional nine passages (47–54 days) under the same culture conditions, iSSCs slowly proliferated and maintained their morphology. Ultimately, a cell type with an ES-like morphology (mSSC) appeared from the iSSC colonies, and two mSSC cell lines were established. The mSSCs had a high proliferative potential in serum-free ES culture medium and have been successfully maintained since their first establishment (> 12 months). We also compared the specific characteristics of iSSCs with those of SSCs and mSSCs using immunocytochemistry, FACS, RT-PCR, DNA methylation, and miRNA analyses. The results suggest that iSSCs represent a morphologically distinct intermediate state with characteristic expression patterns of pluripotency-related genes and miRNAs that arise during the conversion of SSCs into mSSCs. Our results suggest that iSSCs could be a useful model for evaluating and understanding the initiation mechanisms of cell reprogramming.
Similar content being viewed by others
References
Blelloch, R., Wang, Z., Meissner, A., Pollard, S., Smith, A., and Jaenisch, R. (2006). Reprogramming efficiency following somatic cell nuclear transfer is influenced by the differentiation and methylation state of the donor nucleus. Stem Cells 24, 2007–2013.
Brambrink, T., Foreman, R., Welstead, G.G., Lengner, C.J., Wernig, M., Suh, H., and Jaenisch, R. (2008). Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159.
Cavaleri, F.M., Balbach, S.T., Gentile, L., Jauch, A., Bohm-Steuer, B., Han, Y.M., Scholer, H.R., and Boiani, M. (2008). Subsets of cloned mouse embryos and their non-random relationship to development and nuclear reprogramming. Mech. Dev. 125, 153–166.
Chen, C., Ridzon, D.A., Broomer, A.J., Zhou, Z., Lee, D.H., Nguyen, J.T., Barbisin, M., Xu, N.L., Mahuvakar, V.R., Andersen, M.R., et al. (2005). Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 33, e179.
Clarke, D.L., Johansson, C.B., Wilbertz, J., Veress, B., Nilsson, E., Karlstrom, H., Lendahl, U., and Frisen, J. (2000). Generalized potential of adult neural stem cells. Science 288, 1660–1663.
Cowan, C.A., Atienza, J., Melton, D.A., and Eggan, K. (2005). Nuclear reprogramming of somatic cells after fusion with human embryonic stem cells. Science 309, 1369–1373.
Egli, D., Rosains, J., Birkhoff, G., and Eggan, K. (2007). Developmental reprogramming after chromosome transfer into mitotic mouse zygotes. Nature 447, 679–685.
Eisen, M.B., Spellman, P.T., Brown, P.O., and Botstein, D. (1998). Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95, 14863–14868.
Gimble, J.M., Katz, A.J., and Bunnell, B.A. (2007). Adipose-derived stem cells for regenerative medicine. Circ. Res. 100, 1249–1260.
Guan, K., Nayernia, K., Maier, L.S., Wagner, S., Dressel, R., Lee, J.H., Nolte, J., Wolf, F., Li, M., Engel, W., et al. (2006). Pluripotency of spermatogonial stem cells from adult mouse testis. Nature 440, 1199–1203.
Hayashi, K., Chuva de Sousa Lopes, S.M., Kaneda, M., Tang, F., Hajkova, P., Lao, K., O’Carroll, D., Das, P.P., Tarakhovsky, A., Miska, E.A., et al. (2008). MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS ONE 3, e1738.
Hohjoh, H., and Fukushima, T. (2007a). Expression profile analysis of microRNA (miRNA) in mouse central nervous system using a new miRNA detection system that examines hybridization signals at every step of washing. Gene 391, 39–44.
Hohjoh, H., and Fukushima, T. (2007b). Marked change in microRNA expression during neuronal differentiation of human teratocarcinoma NTera2D1 and mouse embryonal carcinoma P19 cells. Biochem. Biophys. Res. Commun. 362, 360–367.
Houbaviy, H.B., Murray, M.F., and Sharp, P.A. (2003). Embryonic stem cell-specific MicroRNAs. Dev. Cell 5, 351–358.
Izadyar, F., Pau, F., Marh, J., Slepko, N., Wang, T., Gonzalez, R., Ramos, T., Howerton, K., Sayre, C., and Silva, F. (2008). Generation of multipotent cell lines from a distinct population of male germ line stem cells. Reproduction 135, 771–784.
Jiang, Y., Jahagirdar, B.N., Reinhardt, R.L., Schwartz, R.E., Keene, C.D., Ortiz-Gonzalez, X.R., Reyes, M., Lenvik, T., Lund, T., Blackstad, M., et al. (2002). Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49.
Kanatsu-Shinohara, M., Ogonuki, N., Inoue, K., Miki, H., Ogura, A., Toyokuni, S., and Shinohara, T. (2003). Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol. Reprod. 69, 612–616.
Kanatsu-Shinohara, M., Inoue, K., Lee, J., Yoshimoto, M., Ogonuki, N., Miki, H., Baba, S., Kato, T., Kazuki, Y., Toyokuni, S., et al. (2004). Generation of pluripotent stem cells from neonatal mouse testis. Cell 119, 1001–1012.
Kanatsu-Shinohara, M., Lee, J., Inoue, K., Ogonuki, N., Miki, H., Toyokuni, S., Ikawa, M., Nakamura, T., Ogura, A., and Shinohara, T. (2008). Pluripotency of a single spermatogonial stem cell in mice. Biol. Reprod. 78, 681–687.
Kim, K.S., Kim, J.S., Lee, M.R., Jeong, H.S., and Kim, J. (2009). A study of microRNAs in silico and in vivo: emerging regulators of embryonic stem cells. FEBS J. 276, 2140–2149.
Lin, S.L., Chang, D.C., Chang-Lin, S., Lin, C.H., Wu, D.T., Chen, D.T., and Ying, S.Y. (2008). Mir-302 reprograms human skin cancer cells into a pluripotent ES-cell-like state. RNA 14, 2115–2124.
Matsui, Y., Zsebo, K., and Hogan, B.L. (1992). Derivation of pluripotential embryonic stem cells from murine primordial germ cells in culture. Cell 70, 841–847.
Oh, S.H., Jung, Y.H., Gupta, M.K., Uhm, S.J., and Lee, H.T. (2009). H19 gene is epigenetically stable in mouse multipotent germline stem cells. Mol. Cells 27, 635–640.
Pasquinelli, A.E., Reinhart, B.J., Slack, F., Martindale, M.Q., Kuroda, M.I., Maller, B., Hayward, D.C., Ball, E.E., Degnan, B., Muller, P., et al. (2000). Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature 408, 86–89.
Seandel, M., James, D., Shmelkov, S.V., Falciatori, I., Kim, J., Chavala, S., Scherr, D.S., Zhang, F., Torres, R., Gale, N.W., et al. (2007). Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature 449, 346–350.
Shamblott, M.J., Axelman, J., Wang, S., Bugg, E.M., Littlefield, J.W., Donovan, P.J., Blumenthal, P.D., Huggins, G.R., and Gearhart, J.D. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. USA 95, 13726–13731.
Solter, D., and Knowles, B.B. (1978). Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. USA 75, 5565–5569.
Suh, M.R., Lee, Y., Kim, J.Y., Kim, S.K., Moon, S.H., Lee, J.Y., Cha, K.Y., Chung, H.M., Yoon, H.S., Moon, S.Y., et al. (2004). Human embryonic stem cells express a unique set of microRNAs. Dev. Biol. 270, 488–498.
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676.
Takamizawa, J., Konishi, H., Yanagisawa, K., Tomida, S., Osada, H., Endoh, H., Harano, T., Yatabe, Y., Nagino, M., Nimura, Y., et al. (2004). Reduced expression of the let-7 microRNAs in human lung cancers in association with shortened postoperative survival. Cancer Res. 64, 3753–3756.
Thomson, J.A., Itskovitz-Eldor, J., Shapiro, S.S., Waknitz, M.A., Swiergiel, J.J., Marshall, V.S., and Jones, J.M. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147.
Wakayama, T., Tabar, V., Rodriguez, I., Perry, A.C., Studer, L., and Mombaerts, P. (2001). Differentiation of embryonic stem cell lines generated from adult somatic cells by nuclear transfer. Science 292, 740–743.
Wilmut, I., Schnieke, A.E., McWhir, J., Kind, A.J., and Campbell, K.H. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813.
Wulczyn, F.G., Smirnova, L., Rybak, A., Brandt, C., Kwidzinski, E., Ninnemann, O., Strehle, M., Seiler, A., Schumacher, S., and Nitsch, R. (2007). Post-transcriptional regulation of the let-7 microRNA during neural cell specification. FASEB J. 21, 415–426.
Yu, J., Vodyanik, M.A., He, P., Slukvin, I.I., and Thomson, J.A. (2006). Human embryonic stem cells reprogram myeloid precursors following cell-cell fusion. Stem Cells 24, 168–176.
Author information
Authors and Affiliations
Corresponding authors
About this article
Cite this article
Kim, H.J., Lee, H.J., Lim, J.J. et al. Identification of an intermediate state as spermatogonial stem cells reprogram to multipotent cells. Mol Cells 29, 519–526 (2010). https://doi.org/10.1007/s10059-010-0064-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-010-0064-5