Abstract
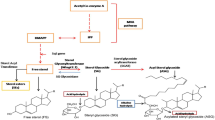
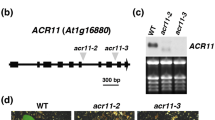
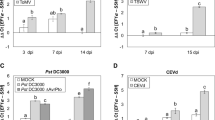
We reported previously that overexpression of a salicylic acid (SA) methyltransferase1 gene from rice (OsBSMT1) or a SA glucosyltransferase1 gene from Arabidopsis thaliana (AtSAGT1) leads to increased susceptibility to Pseudomonas syringae due to reduced SA levels. To further examine their roles in the defense responses, we assayed the transcript levels of AtBSMT1 or AtSAGT1 in plants with altered levels of SA and/or other defense components. These data showed that AtSAGT1 expression is regulated partially by SA, or nonexpressor of pathogenesis related protein1, whereas AtBSMT1 expression was induced in SA-deficient mutant plants. In addition, we produced the transgenic Arabidopsis plants with RNAi-mediated inhibition of AtSAGT1 and isolated a null mutant of AtBSMT1 and then analyzed their phenotypes. A T-DNA insertion mutation in the AtBSMT1 resulted in reduced methyl salicylate (MeSA) levels upon P. syringae infection. However, accumulation of SA and glucosyl SA was similar in both the atbsmt1 and wild-type plants, indicating the presence of another SA methyltransferase or an alternative pathway for MeSA production. The AtSAGT1-RNAi line exhibited no altered phenotypes upon pathogen infection, compared to wild-type plants, suggesting that (an)other SA glucosyltransferase(s) in Arabidopsis plants may be important for the pathogenesis of P. syringae.
Similar content being viewed by others
References
Attaran, E., Zeier, T.E., Griebel, T., and Zeier J. (2009). Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21, 954–971.
Baldwin, I.T., Halitschke, R., Paschold, A., von Dahl, C.C., and Preston, C.A. (2006). Volatile signaling in plant-plant interactions: “talking trees” in the genomics era. Science 311, 812–815.
Cao, H., Bowling, S.A., Gordon, S., and Dong, X. (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583–1592.
Chen, F., D’Auria, J.C., Tholl, D., Ross, J.R., Gershenzon, J., Noel, J.P., and Pichersky, E. (2003). An Arabidopsis thaliana gene for methylsalicylate biosynthesis, identified by a biochemical genomics approach, has a role in defense. Plant J. 36, 577–588.
Dean, J., and Delaney, S. (2008). Metabolism of salicylic acid in wild-type, ugt74f1 and ugt74f2 glucosyltransferase mutants of Arabidopsis thaliana. Physiol. Plant. 132, 417–425.
Dean, J., Mohammed, L.A., and Fitzpatrick, T. (2005). The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221, 287–296.
Delaney, T.P., Uknes, S., Vernooij, B., Friedrich, L., Weymann, K., Negrotto, D., Gaffney, T., Gut-Rella, M., Kessmann, H., Ward, E., et al. (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247–1250.
Edwards, R. (1994). Conjugation and metabolism of salicylic acid in tobacco. J. Plant Physiol. 143, 609–614.
Engelberth, J., Schmelz, E.A., Alborn, H.T., Cardoza, Y.J., Huang, J., and Tumlinson, J.H. (2003). Simulaneous quantification of jasmonic acid and salicylic acid in plants by vapor-phase extraction and gas chromatography-chemical ionization-mass spectrometry. Anal. Biochem. 312, 242–250.
Gaffney, T., Friedrich, L., Vernooij, B., Negrotto, D., Nye, G., Uknes, S., Ward, E., Kessmann, H., and Ryals, J. (1993). Requirement of salicylic acid for the induction systemic acquired resistance. Science 261, 754–756.
Kim, M.G., Kim, S.Y., Kim, W.Y., Mackey, D., and Lee, S.Y. (2008). Responses of Arabidopsis thaliana to challenge by Pseudomonas syringae. Mol. Cells 25, 323–331.
Koo, Y.J., Kim, M.A., Kim, E.H., Song, J.T., Jung, C., Moon, J.-K., Kim, J.-H., Seo, H.S., Song, S.I., Kim, J.-K., et al. (2007). Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant Mol. Biol. 64, 1–15.
Kroczek, R.A., and Siebert, E. (1990). Optimization of Northern analysis by vaccum-blotting, RNA transfer, visualization and ultraviolet fixation. Anal. Biochem. 184, 90–95.
Lee, H.-I., and Raskin, I. (1998). Glucosylation of salicylic acid in Nicotiana tabacum cv. Xanthi-nc. Phytopathology 88, 692–697.
Lee, H.-I., Leon, J., and Raskin, I. (1995). Biosynthesis and metabolism of salicylic acid. Proc. Natl. Acad. Sci. USA 92, 4076–4079.
Loake, G., and Grant, M. (2007). Salicyllic acid in plant defense-the players and protagonists. Curr. Opin. Plant Biol. 10, 466–472.
Lim, E.-K., Doucet, C.J., Li, Y., Elias, L., Worrall, D., Spencer, S.P., Ross, J., and Bowles, D.J. (2002). The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J. Biol. Chem. 277, 586–592.
Park, S.W., Kaimoyo, E., Kumar, D., Mosher, S., and Klessig, D.F. (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318, 113–116.
Rate, D.N., and Greenberg, J.T. (2001). The Arabidopsis aberrant growth and death2 mutant shows resistance to Pseudomonas syringae and reveals a role for NPR1 in suppressing hypersensitive cell death. Plant J. 27, 203–211.
Rate, D.N., Cuenca, J.V., Bowman, G.R., and Greenberg, J.T. (1999). The gain-of-function Arabidopsis acd6 mutant reveals novel regulation and function of the salicylic acid signaling pathway in controlling cell death, defense, and cell growth. Plant Cell 11, 1695–1708.
Seskar, M., Shulaev, V., and Raskin, I. (1998). Endogenous methyl salicylate in pathogen-inoculated tobacco plants. Plant Physiol. 116, 387–392.
Shulaev, V., Silverman, P., and Raskin, I. (1997). Airborne signaling by methyl salicylate in plant pathogen resistance. Nature 385, 718–721.
Song, J.T. (2006). Induction of a salicylic acid glucosyltransferase, AtSGT1, is an early disease response in Arabidopsis thaliana. Mol. Cells 22, 233–238.
Song, J.T., Koo, Y.J., Seo, H.S., Kim, M.C., Choi, Y.D., and Kim, J.H. (2008). Overexpression of AtSGT1, an Arabidopsis salicylic acid glucosyltransferase, leads to increased susceptibility to Pseudomonas syringae. Phytochemistry 69, 1128–1134.
Vlot, A.C., Liu, P.P., Cameron, R.K., Park, S.W., Yang, Y., Kumar, D., Zhou, F., Padukkavidana, T., Gustafsson, C., Pichersky, E., et al. (2008). Identification of likely orthologs of tobacco salicylic acid-binding protein 2 and their role in systemic acquired resistance in Arabidopsis thaliana. Plant J. 56, 445–456.
Wildermuth, M.C., Dewdney, J., Wu, G., and Ausubel F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defense. Nature 414, 562–565.
Zhou, N., Tootle, T.L., Tsui, F., Klessig, D.F., and Glazebrook, J. (1998). PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10, 1021–1030.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Song, J.T., Koo, Y.J., Park, JB. et al. The expression patterns of AtBSMT1 and AtSAGT1 encoding a salicylic acid (SA) methyltransferase and a SA glucosyltransferase, respectively, in Arabidopsis plants with altered defense responses. Mol Cells 28, 105–109 (2009). https://doi.org/10.1007/s10059-009-0108-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10059-009-0108-x