Abstract:
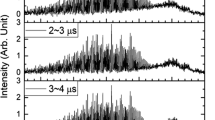
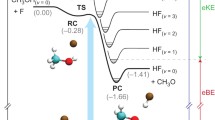
Applying the two photon laser induced fluorescence technique for nascent state resolved ClO() detection, the reaction dynamics of Cl+O ClO+O2 is investigated. The ClO product is formed in its electronic ground state ClO(). A complete product state analysis in terms of vibration, rotation, spin-orbit and -states indicates that nascent ClO radicals are formed in v =0-6 vibrational states peaking at v =3. The ClO fragment shows a moderate rotational excitation, described by a Boltzmann distribution with a temperature parameter of 1300 K 200 K. The spin orbit ratio of :. Most of the excess energy is released as translational energy or as internal energy of the O2 product. By comparing our results with the trajectory studies of Farantos and Murrell, we favour a reaction mechanism, where the transition complex is planar containing an essentially linear OOCl group. In order to determine the possible influence of vibrationally excited ClO on other trace components of the atmosphere, especially the reaction ClO(v >0)+ O3, a rough estimate of the vibrational relaxation rate of ClO with the major atmospheric collision partner, N2, has been performed. A measurement of the vibrational distribution of ClO at different N2 pressures indicates a mean vibrational relaxation rate of .
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 27 February 1998 / Revised: 1st April 1998 / Accepted: 15 April 1998
Rights and permissions
About this article
Cite this article
Baumgärtel, S., Delmdahl, R., Gericke, KH. et al. Reaction dynamics of Cl O ClO O . Eur. Phys. J. D 4, 199–205 (1998). https://doi.org/10.1007/s100530050200
Issue Date:
DOI: https://doi.org/10.1007/s100530050200