Abstract
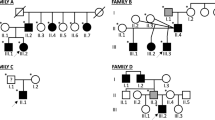
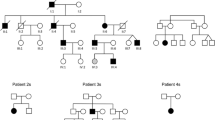
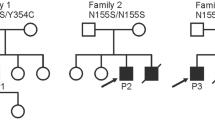
Emery–Dreifuss muscular dystrophy (EDMD) is characterised by early-onset joint contractures, progressive muscular weakness and wasting and late-onset cardiac disease. The more common X-linked recessive form of EDMD is caused by mutations in either EMD (encoding emerin) or FHL1 (encoding four and a half LIM domains 1), while mutations in LMNA (encoding lamin A/C), SYNE1 (encoding nesprin-1) and SYNE2 (encoding nesprin-2) lead to autosomal dominant forms of the condition. Here, we identify a three-generation family with an extended EDMD phenotype due to a novel indel mutation in FHL1 that differentially affects the relative expression of the three known transcript isoforms produced from this locus. The additional phenotypic manifestations in this family—proportionate short stature, facial dysmorphism, pulmonary valvular stenosis, thoracic scoliosis, brachydactyly, pectus deformities and genital abnormalities—are reminiscent of phenotypes seen with dysregulated Ras–mitogen-activated protein kinase (RAS-MAPK) signalling [Noonan syndrome (NS) and related disorders]. The misexpression of FHL1 transcripts precipitated by this mutation, together with the role of FHL1 in the regulation of RAS-MAPK signalling, suggests that this mutation confers a complex phenotype through both gain- and loss-of-function mechanisms. This indel mutation in FHL1 broadens the spectrum of FHL1-related disorders and implicates it in the pathogenesis of NS spectrum disorders.



Similar content being viewed by others
References
Emery AE (2000) Emery–Dreifuss muscular dystrophy—a 40 year retrospective. Neuromuscul Disord 10:228–232
Bione S, Maestrini E, Rivella S, Mancini M, Regis S, Romeo G, Toniolo D (1994) Identification of a novel X-linked gene responsible for Emery–Dreifuss muscular dystrophy. Nat Genet 8:323–327
Gueneau L, Bertrand AT, Jais JP, Salih MA, Stojkovic T, Wehnert M, Hoeltzenbein M, Spuler S, Saitoh S, Verschueren A, Tranchant C, Beuvin M, Lacene E, Romero NB, Heath S, Zelenika D, Voit T, Eymard B, Ben Yaou R, Bonne G (2009) Mutations of the FHL1 gene cause Emery–Dreifuss muscular dystrophy. Am J Hum Genet 85:338–353
Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K (1999) Mutations in the gene encoding lamin A/C cause autosomal dominant Emery–Dreifuss muscular dystrophy. Nat Genet 21:285–288
Zhang QP, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi QJ, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM (2007) Nesprin-1 and -2 are involved in the pathogenesis of Emery–Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 16:2816–2833
Liang L, Zhang HW, Liang J, Niu XL, Zhang SZ, Feng L, Liang YM, Han H (2008) KyoT3, an isoform of murine FHL1, associates with the transcription factor RBP-J and represses the RBP-J-mediated transactivation. Biochim Et Biophys Acta-Gene Regul Mech 1779:805–810
Qin HY, Du DW, Zhu YT, Li JF, Feng L, Liang YM, Han H (2005) The PcG protein HPC2 inhibits RBP-J-mediated transcription by interacting with LIM protein KyoT2. FEBS Lett 579:1220–1226
Qin HY, Wang JS, Liang YM, Taniguchi Y, Tanigaki K, Han H (2004) RING1 inhibits transactivation of RBP-J by Notch through interaction with LIM protein KyoT2. Nucleic Acids Res 32:1492–1501
Taniguchi Y, Furukawa T, Tun T, Han H, Honjo T (1998) LIM protein KyoT2 negatively regulates transcription by association with the RBP-J DNA-binding protein. Mol Cell Biol 18:644–654
Wang J, Qin H, Liang J, Zhu Y, Liang L, Zheng M, Han H (2007) The transcriptional repression activity of KyoT2 on the Notch/RBP-J pathway is regulated by PIAS1-catalyzed SUMOylation. FEBS J 274:218–218
McGrath MJ, Cottle DL, Nguyen MA, Dyson JM, Coghill ID, Robinson PA, Holdsworth M, Cowling BS, Hardeman EC, Mitchell CA, Brown S (2006) Four and a half LIM protein 1 binds myosin-binding protein C and regulates myosin filament formation and sarcomere assembly. J Biol Chem 281:7666–7683
Sheikh F, Raskin A, Chu PH, Lange S, Domenighetti AA, Zheng M, Liang XQ, Zhang T, Yajima T, Gu Y, Dalton ND, Mahata SK, Dorn GW, Heller-Brown J, Peterson KL, Omens JH, McCulloch AD, Chen J (2008) An FHL1-containing complex within the cardiomyocyte sarcomere mediates hypertrophic biomechanical stress responses in mice. J Clin Invest 118:3870–3880
Lee SMY, Tsui SKW, Chan KK, Garcia-Barcelo M, Waye MMY, Fung KP, Liew CC, Lee CY (1998) Chromosomal mapping, tissue distribution and cDNA sequence of four-and-a-half LIM domain protein 1 (FHL1). Gene 216:163–170
Lee SMY, Li HY, Ng EKO, Or SMW, Chan KK, Kotaka M, Chim SSC, Tsui SKW, Waye MMY, Fung KP, Lee CY (1999) Characterization of a brain-specific nuclear LIM domain protein (FHL1B) which is an alternatively spliced variant of FHL1. Gene 237:253–263
Brown S, McGrath MJ, Ooms LM, Gurung R, Maimone MM, Mitchell CA (1999) Characterization of two isoforms of the skeletal muscle LIM protein 1, SLIM1. Localization of SLIM1 at focal adhesions and the isoform slimmer in the nucleus of myoblasts and cytoplasm of myotubes suggests distinct roles in the cytoskeleton and in nuclear-cytoplasmic communication. J Biol Chem 274:27083–27091
Ng EKO, Lee SMY, Li HY, Ngai SM, Tsui SKW, Waye MMY, Lee CY, Fung KP (2001) Characterization of tissue-specific LIM domain protein (FHL1C) which is an alternatively spliced isoform of a human LIM-only protein (FHL1). J Cell Biochem 82:1–10
Friedrich FW, Wilding BR, Reischmann S, Crocini C, Lang P, Charron P, Muller OJ, McGrath MJ, Vollert I, Hansen A, Linke WA, Hengstenberg C, Bonne G, Morner S, Wichter T, Madeira H, Arbustini E, Eschenhagen T, Mitchell CA, Isnard R, Carrier L (2012) Evidence for FHL1 as a novel disease gene for isolated hypertrophic cardiomyopathy. Hum Mol Genet 21:3237–3254
Schessl J, Columbus A, Hu Y, Zou Y, Voit T, Goebel HH, Bonnemann CG (2010) Familial reducing body myopathy with cytoplasmic bodies and rigid spine revisited: identification of a second LIM domain mutation in FHL1. Neuropediatrics 41:43–46
Schessl J, Taratuto AL, Sewry C, Battini R, Chin SS, Maiti B, Dubrovsky AL, Erro MG, Espada G, Robertella M, Saccoliti M, Olmos P, Bridges LR, Standring P, Hu Y, Zou Y, Swoboda KJ, Scavina M, Goebel HH, Mitchell CA, Flanigan KM, Muntoni F, Bonnemann CG (2009) Clinical, histological and genetic characterization of reducing body myopathy caused by mutations in FHL1. Brain 132:452–464
Schessl J, Zou Y, McGrath MJ, Cowling BS, Maiti B, Chin SS, Sewry C, Battini R, Hu Y, Cottle DL, Rosenblatt M, Spruce L, Ganguly A, Kirschner J, Judkins AR, Golden JA, Goebel HH, Muntoni F, Flanigan KM, Mitchell CA, Bonnemann CG (2008) Proteomic identification of FHL1 as the protein mutated in human reducing body myopathy. J Clin Invest 118:904–912
Shalaby S, Hayashi YK, Nonaka I, Noguchi S, Nishino I (2009) Novel FHL1 mutations in fatal and benign reducing body myopathy. Neurology 72:375–376
Quinzi CM, Vu TH, Min KC, Tanji K, Barral S, Grewal RP, Kattah A, Camano P, Otaegui D, Kunimatsu T, Blake DM, Wilhelmsen KC, Rowland LP, Hays AP, Bonilla E, Hirano M (2008) X-linked dominant scapuloperoneal myopathy is due to a mutation in the gene encoding four-and-a-half-LIM protein 1. Am J Hum Genet 82:208–213
Chen DH, Raskind WH, Parson WW, Sonnen JA, Vu T, Zheng Y, Matsushita M, Wolff J, Lipe H, Bird TD (2010) A novel mutation in FHL1 in a family with X-linked scapuloperoneal myopathy: phenotypic spectrum and structural study of FHL1 mutations. J Neurol Sci 296:22–29
Windpassinger C, Schoser B, Straub V, Hochmeister S, Noor A, Lohberger B, Farra N, Petek E, Schwarzbraun T, Ofner L, Loscher WN, Wagner K, Lochmuller H, Vincent JB, Quasthoff S (2008) An X-linked myopathy with postural muscle atrophy and generalized hypertrophy, termed XMPMA, is caused by mutations in FHL1. Am J Hum Genet 82:88–99
Schoser B, Goebel HH, Janisch I, Quasthoff S, Rother J, Bergmann M, Muller-Felber W, Windpassinger C (2009) Consequences of mutations within the C terminus of the FHL1 gene. Neurology 73:543–551
Shalaby S, Hayashi YK, Goto K, Ogawa M, Nonaka I, Noguchi S, Nishino I (2008) Rigid spine syndrome caused by a novel mutation in four-and-a-half LIM domain 1 gene (FHL1). Neuromuscul Disord 18:959–961
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Jorge AA, Malaquias AC, Arnhold IJ, Mendonca BB (2009) Noonan syndrome and related disorders: a review of clinical features and mutations in genes of the RAS/MAPK pathway. Horm Res 71:185–193
Tartaglia M, Zampino G, Gelb BD (2010) Noonan syndrome: clinical aspects and molecular pathogenesis. Mol Syndromol 1:2–26
Tartaglia M, Gelb BD, Zenker M (2011) Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab 25:161–179
Miskinyte S, Butler MG, Herve D, Sarret C, Nicolino M, Petralia JD, Bergametti F, Arnould M, Pham VN, Gore AV, Spengos K, Gazal S, Woimant F, Steinberg GK, Weinstein BM, Tournier-Lasserve E (2011) Loss of BRCC3 deubiquitinating enzyme leads to abnormal angiogenesis and is associated with syndromic moyamoya. Am J Hum Genet 88:718–728
Cowling BS, Cottle DL, Wilding BR, D'Arcy CE, Mitchell CA, McGrath MJ (2011) Four and a half LIM protein 1 gene mutations cause four distinct human myopathies: a comprehensive review of the clinical, histological and pathological features. Neuromuscul Disord 21:237–251
Schessl J, Feldkirchner S, Kubny C, Schoser B (2011) Reducing body myopathy and other FHL1-related muscular disorders. Semin Pediatr Neurol 18:257–263
Cottle DL, McGrath MJ, Wilding BR, Cowling BS, Kane JM, D'Arcy CE, Holdsworth M, Hatzinisiriou I, Prescott M, Brown S, Mitchell CA (2009) SLIMMER (FHL1B/KyoT3) interacts with the proapoptotic protein Siva-1 (CD27BP) and delays skeletal myoblast apoptosis. J Biol Chem 284:26964–26977
Poparic I, Schreibmayer W, Schoser B, Desoye G, Gorischek A, Miedl H, Hochmeister S, Binder J, Quasthoff S, Wagner K, Windpassinger C, Malle E (2011) Four and a half LIM protein 1C (FHL1C): a binding partner for voltage-gated potassium channel K(v1.5). PLoS One 6:e26524
Yang Z, Browning CF, Hallaq H, Yermalitskaya L, Esker J, Hall MR, Link AJ, Ham AJL, McGrath MJ, Mitche CA, Murray KT (2008) Four and a half LIM protein 1: a partner for KCNA5 in human atrium. Cardiovasc Res 78:449–457
Yang J, Moravec CS, Sussman MA, DiPaola NR, Fu D, Hawthorn L, Mitchell CA, Young JB, Francis GS, McCarthy PM, Bond M (2000) Decreased SLIM1 expression and increased gelsolin expression in failing human hearts measured by high-density oligonucleotide arrays. Circulation 102:3046–3052
Hwang DM, Dempsey AA, Lee CY, Liew CC (2000) Identification of differentially expressed genes in cardiac hypertrophy by analysis of expressed sequence tags. Genomics 66:1–14
Lim DS, Roberts R, Marian AJ (2001) Expression profiling of cardiac genes in human hypertrophic cardiomyopathy: insight into the pathogenesis of phenotypes. J Am Coll Cardiol 38:1175–1180
Chu PH, Ruiz-Lozano P, Zhou Q, Cai C, Chen J (2000) Expression patterns of FHL/SLIM family members suggest important functional roles in skeletal muscle and cardiovascular system. Mech Dev 95:259–265
Gaussin V, Tomlinson JE, Depre C, Engelhardt S, Antos CL, Takagi G, Hein L, Topper JN, Liggett SB, Olson EN, Lohse MJ, Vatner SF, Vatner DE (2003) Common genomic response in different mouse models of beta-adrenergic-induced cardiomyopathy. Circulation 108:2926–2933
Kondoh K, Sunadome K, Nishida E (2007) Notch signaling suppresses p38 MAPK activity via induction of MKP-1 in myogenesis. J Biol Chem 282:3058–3065
Boutros T, Chevet E, Metrakos P (2008) Mitogen-activated protein (MAP) kinase/MAP kinase phosphatase regulation: roles in cell growth, death, and cancer. Pharmacol Rev 60:261–310
Owens DM, Keyse SM (2007) Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene 26:3203–3213
Sun H, Charles CH, Lau LF, Tonks NK (1993) MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell 75:487–493
Ganesan V, Kirkham FJ (1997) Noonan syndrome and moyamoya. Pediatr Neurol 16:256–258
Hung PC, Wang HS, Wong AM (2011) Moyamoya syndrome in a child with Noonan syndrome. Pediatr Neurol 45:129–131
Tang KT, Yang W, Wong J, Lee KY (1999) Noonan syndrome associated with moyamoya disease: report of one case. Acta Paediatr Taiwan 40:274–276
Yamashita Y, Kusaga A, Koga Y, Nagamitsu S, Matsuishi T (2004) Noonan syndrome, moyamoya-like vascular changes, and antiphospholipid syndrome. Pediatr Neurol 31:364–366
Digilio MC, Lepri F, Baban A, Dentici ML, Versacci P, Capolino R, Ferese R, De Luca A, Tartaglia M, Marino B, Dallapiccola B (2011) RASopathies: clinical diagnosis in the first year of life. Mol Syndromol 1:282–289
Fabretto A, Kutsche K, Harmsen MB, Demarini S, Gasparini P, Fertz MC, Zenker M (2010) Two cases of Noonan syndrome with severe respiratory and gastroenteral involvement and the SOS1 mutation F623I. Eur J Med Genet 53:322–324
Yellon RF (1997) Complications following airway surgery in Noonan syndrome. Arch Otolaryngol Head Neck Surg 123:1341–1343
Digilio MC, Sarkozy A, Capolino R, Chiarini Testa MB, Esposito G, de Zorzi A, Cutrera R, Marino B, Dallapiccola B (2008) Costello syndrome: clinical diagnosis in the first year of life. Eur J Pediatr 167:621–628
Gripp KW, Hopkins E, Sol-Church K, Stabley DL, Axelrad ME, Doyle D, Dobyns WB, Hudson C, Johnson J, Tenconi R, Graham GE, Sousa AB, Heller R, Piccione M, Corsello G, Herman GE, Tartaglia M, Lin AE (2011) Phenotypic analysis of individuals with Costello syndrome due to HRAS p.G13C. Am J Med Genet Part A 155A:706–716
Kawame H, Matsui M, Kurosawa K, Matsuo M, Masuno M, Ohashi H, Fueki N, Aoyama K, Miyatsuka Y, Suzuki K, Akatsuka A, Ochiai Y, Fukushima Y (2003) Further delineation of the behavioral and neurologic features in Costello syndrome. Am J Med Genet Part A 118A:8–14
Acknowledgments
The authors thank the family for their participation in this research. This work was funded by Curekids New Zealand.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tiffin, H.R., Jenkins, Z.A., Gray, M.J. et al. Dysregulation of FHL1 spliceforms due to an indel mutation produces an Emery–Dreifuss muscular dystrophy plus phenotype. Neurogenetics 14, 113–121 (2013). https://doi.org/10.1007/s10048-013-0359-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10048-013-0359-8