Abstract
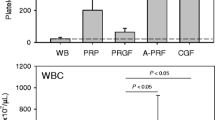
Platelet-rich plasma (PRP) is blood plasma that has been enriched with platelets. It holds promise for clinical use in areas such as wound healing and regenerative medicine, including bone regeneration. This study characterized the composition of PRP produced by seven commercially available separation systems (JP200, GLO PRP, Magellan Autologous Platelet Separator System, KYOCERA Medical PRP Kit, SELPHYL, MyCells, and Dr. Shin’s System THROMBO KIT) to evaluate the platelet, white blood cell, red blood cell, and growth factor concentrations, as well as platelet-derived growth factor-AB (PDGF-AB), transforming growth factor beta-1 (TGF-β1), and vascular endothelial growth factor (VEGF) concentrations. PRP prepared using the Magellan Autologous Platelet Separator System and the KYOCERA Medical PRP Kit contained the highest platelet concentrations. The mean PDGF-AB concentration of activated PRP was the highest from JP200, followed by the KYOCERA Medical PRP Kit, Magellan Autologous Platelet Separator System, MyCells, and GLO PRP. TGF-β1 and VEGF concentrations varied greatly among individual samples, and there was almost no significant difference among the different systems, unlike for PDGF. The SELPHYL system produced PRP with low concentrations of both platelets and growth factors. Commercial PRP separation systems vary widely, and familiarity with their individual advantages is important to extend their clinical application to a wide variety of conditions.


Similar content being viewed by others
References
Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–60.
Kakudo N, Morimoto N, Kushida S, Ogawa T, Kusumoto K. Platelet-rich plasma releasate promotes angiogenesis in vitro and in vivo. Med Mol Morphol (in press).
Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96.
Salcido RS. Autologous platelet-rich plasma in chronic wounds. Adv Skin Wound Care. 2013;26:248.
Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46.
de Almeida AM, Demange MK, Sobrado MF, Rodrigues MB, Pedrinelli A, Hernandez AJ. Patellar tendon healing with platelet-rich plasma: a prospective randomized controlled trial. Am J Sports Med. 2012;40:1282–8.
Man D, Plosker H, Winland-Brown JE. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–237, discussion 238–229.
Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–71.
Weibrich G, Kleis WK, Hafner G. Growth factor levels in the platelet-rich plasma produced by 2 different methods: curasan-type PRP kit versus PCCS PRP system. Int J Oral Maxillofac Implant. 2002;17:184–90.
Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: a review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147e–59e.
Gonshor A. Technique for producing platelet-rich plasma and platelet concentrate: background and process. Int J Periodontics Restor Dent. 2002;22:547–57.
Hosgood G. Wound healing: the role of platelet-derived growth factor and transforming growth factor beta. Vet Surg. 1993;22:490–5.
Civinini R, Nistri L, Martini C, Redl B, Ristori G, Innocenti M. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013;10:26–9.
Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–80.
Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8.
Kakudo N, Kushida S, Ogura T, Hara T, Suzuki K, Kusumoto K. The use of autologous platelet-rich plasma in the treatment of intractable skin ulcer: a case series. OJRM. 2012;1:29–32.
Vang SN, Brady CP, Christensen KA, Allen KR, Anderson JE, Isler JR, Holt DW, Smith LM. Autologous platelet gel in coronary artery bypass grafting: effects on surgical wound healing. J Extra Corpor Technol. 2007;39:31–8.
Hom DB, Linzie BM, Huang TC. The healing effects of autologous platelet gel on acute human skin wounds. Arch Facial Plast Surg. 2007;9:174–83.
Zavadil DP, Satterlee CC, Costigan JM, Holt DW, Shostrom VK. Autologous platelet gel and platelet-poor plasma reduce pain with total shoulder arthroplasty. J Extra Corpor Technol. 2007;39:177–82.
Brady C, Vang S, Christensen K, Isler J, Vollstedt K, Holt D. Use of autologous platelet gel in bariatric surgery. J Extra Corpor Technol. 2006;38:161–4.
Sclafani AP. Applications of platelet-rich fibrin matrix in facial plastic surgery. Facial Plast Surg. 2009;25:270–6.
Sclafani AP. Platelet-rich fibrin matrix for improvement of deep nasolabial folds. J Cosmet Dermatol. 2010;9:66–71.
Sclafani AP, McCormick SA. Induction of dermal collagenesis, angiogenesis, and adipogenesis in human skin by injection of platelet-rich fibrin matrix. Arch Facial Plast Surg. 2012;14:132–6.
Conflict of interest
None of the authors has a financial interest in any of the products, devices, or drugs mentioned in this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kushida, S., Kakudo, N., Morimoto, N. et al. Platelet and growth factor concentrations in activated platelet-rich plasma: a comparison of seven commercial separation systems. J Artif Organs 17, 186–192 (2014). https://doi.org/10.1007/s10047-014-0761-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-014-0761-5