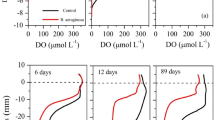
Abstract
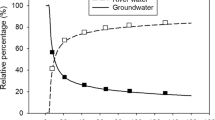
The behavior of iron (Fe) in clayey aquitards has a significant effect on the groundwater environment. However, the release processes and impact of Fe within clayey sediments during compaction remain unknown. Two groups of simulation experiments were carried out to demonstrate the migration and transformation mechanisms of Fe during clayey sediment compaction. Experiment A, which simulated a natural deposition condition, revealed that pressurization changed the reaction environment from oxidative to reductive by isolating oxygen. Oxidation of ferrous ions was followed by reduction dissolution of poorly crystalline Fe (III) and crystalline Fe (III) oxides. Under the microbial utilization of organic matter, the main transformation process of sediment Fe was the dissimilatory reduction of poorly crystalline Fe (III) oxides. The total Fe concentration in pore water was 0.09–11.61 mg/L, with ferrous ions predominating among the Fe species. The lower moisture content (\(\text{<}\)~36%) in the later stage of compaction inhibited the dissimilatory reduction of Fe (III), and the formation of Fe (II) minerals resulted in a decrease in Fe concentration. Experiment B, which simulated an artificial compaction state, revealed that the sediment Fe was primarily released by physical dissolution because of changes in pore structure and solubility. The concentration of total Fe in pore water was 0.02–1.96 mg/L, with a significant increase in response to a rapid increase in pressure. According to the estimates in the Chen Lake wetland (eastern China), the contribution of clay pore water release accounted for 19.9–31.9% of the average Fe concentration in groundwater during natural deposition.
Résumé
Le comportement du fer (Fe) dans les aquitards argileux a un effet significatif sur l’environnement des eaux souterraines. Cependant, les processus de libération et l’impact du Fe dans les sédiments argileux pendant le compactage restent inconnus. Deux groupes d’expériences de simulation ont été réalisés pour démontrer les mécanismes de migration et de transformation du Fe pendant le compactage des sédiments argileux. L’expérience A, qui simulait des conditions de dépôt naturelles, a révélé que la pressurisation modifiait l’environnement réactionnel d’oxydant à réducteur en isolant l’oxygène. L’oxydation des ions ferreux a été suivie par la dissolution réductrice du Fe (III) mal cristallisé et des oxydes de Fe (III) cristallisés. Dans le cadre de l’utilisation microbienne de la matière organique, le principal processus de transformation du Fe des sédiments était la réduction dissimilaire des oxydes de Fe (III) peu cristallins. La concentration totale du Fe dans l’eau interstitielle était de 0.09 à 11.61 mg/L, les ions ferreux prédominant parmi les espèces de Fe. La teneur en humidité plus faible (\(\text{<} \,\)~36%) au cours de la dernière étape du compactage a inhibé la réduction dissimulatrice de Fe (III), et la formation de minéraux de Fe (II) a entraîné une diminution de la concentration de Fe. L’expérience B, qui simulait un état de compactage artificiel, a révélé que le sédiment Fe était principalement libéré par dissolution physique en raison de changements dans la structure porale et de la solubilité. La concentration de Fe total dans l’eau interstitielle était de 0.02 à 1.96 mg/L, avec une augmentation significative en réponse à une augmentation rapide de la pression. Selon les estimations faites pour la zone humide de la Chen (partie orientale de la Chine), la contribution d’eau interstitielle libérée par l’argile représentait 19.9 à 31.9% de la concentration moyenne de Fe des eaux souterraines lors du processus naturel de dépôt.
Resumen
El comportamiento del hierro (Fe) en acuíferos arcillosos tiene un efecto significativo en el entorno de las aguas subterráneas. Sin embargo, los procesos de liberación y el impacto del Fe dentro de los sedimentos arcillosos durante la compactación siguen siendo desconocidos. Se llevaron a cabo dos grupos de experimentos de simulación para demostrar los mecanismos de migración y transformación del Fe durante la compactación de sedimentos arcillosos. El experimento A, que simuló una condición de sedimentación natural, reveló que la presurización cambió el ambiente de reacción de oxidativo a reductivo aislando el oxígeno. La oxidación de iones ferrosos fue seguida por la disolución reductora de Fe (III) poco cristalino y óxidos de Fe (III) cristalinos. Bajo la utilización microbiana de materia orgánica, el principal proceso de transformación del Fe del sedimento fue la reducción disimilatoria de óxidos de Fe (III) pobremente cristalinos. La concentración total de Fe en el agua de poros fue de 0.09–11.61 mg/L, predominando los iones ferrosos entre las especies de Fe. El menor contenido de humedad (< ~36%) en la última etapa de compactación inhibió la reducción disimilatoria del Fe (III), y la formación de minerales de Fe (II) dio lugar a una disminución de la concentración de Fe. El experimento B, que simuló un estado de compactación artificial, reveló que el Fe del sedimento se liberó principalmente por disolución física debido a los cambios en la estructura de los poros y la solubilidad. La concentración de Fe total en el agua de los poros fue de 0.02–1.96 mg/L, con un aumento significativo en respuesta a un rápido incremento de la presión. Según las estimaciones realizadas en el humedal del lago Chen (este de China), la contribución de la liberación de agua de poros de arcilla supuso entre el 19.9 y el 31.9% de la concentración media de Fe en las aguas subterráneas durante la sedimentación natural.
摘要
黏土弱透水层中铁(Fe)的行为对地下水环境有着显著影响。然而, 黏土沉积物压实过程中铁的释放过程及其影响尚不清楚。为了展示黏土沉积物压实过程中铁的迁移转化机制, 进行了两组模拟实验。实验A模拟了自然沉积条件, 结果显示压力作用通过隔绝氧气, 将反应环境由氧化转变为还原环境。继而发生了铁离子的氧化, 以及弱结晶铁(Fe (III))和结晶性铁(Fe (III))氧化物的还原溶解。在微生物对有机质的利用下,沉积物中铁的主要转化过程是弱结晶铁(Fe (III))氧化物的异化还原。孔隙水中的总铁浓度为0.09−11.61 mg/L, 其中亚铁是主要的铁形态。在压实后期, 较低的含水率(<36%)抑制了铁 (III) 的异化还原, 结合铁 (II) 矿物的形成导致铁浓度降低。实验B模拟了人为压实条件, 结果发现沉积物中的铁主要是通过孔隙结构和溶解度变化导致的物理溶解而释放。孔隙水中的总铁浓度为0.02−1.96 mg/L, 而且随着压力的快速升高而显著增加。根据对中国东部沉湖湿地的估算, 自然沉积过程中黏土孔隙水释放对地下水中铁的贡献占平均铁浓度的19.9−31.9%。
Resumo
O comportamento do ferro (Fe) em aquitardos argilosos tem um efeito significativo no ambiente das águas subterrâneas. No entanto, os processos de liberação e o impacto do Fe nos sedimentos argilosos durante a compactação permanecem desconhecidos. Dois grupos de experimentos de simulação foram realizados para demonstrar os mecanismos de migração e transformação do Fe durante a compactação de sedimentos argilosos. O experimento A, que simulou uma condição natural de deposição, revelou que a pressurização mudou o ambiente de reação de oxidativo para redutor, isolando o oxigênio. A oxidação de íons ferrosos foi seguida pela dissolução redutora de óxidos de Fe (III) pouco cristalinos e de Fe (III) cristalinos. Sob a utilização microbiana da matéria orgânica, o principal processo de transformação do Fe sedimentar foi a redução dissimilatória de óxidos de Fe (III) pouco cristalinos. A concentração total de Fe na água dos poros foi de 0.09–11.61 mg/L, com íons ferrosos predominando entre as espécies de Fe. O menor teor de umidade (< ~36%) na fase posterior de compactação inibiu a redução dissimilatória de Fe (III), e a formação de minerais de Fe (II) resultou em uma diminuição na concentração de Fe. O experimento B, que simulou um estado de compactação artificial, revelou que o Fe do sedimento foi liberado principalmente por dissolução física devido a mudanças na estrutura dos poros e na solubilidade. A concentração de Fe total na água dos poros foi de 0.02–1.96 mg/L, com um aumento significativo em resposta a um rápido aumento na pressão. De acordo com as estimativas na zona húmida do Lago Chen (leste da China), a contribuição da libertação de água dos poros de argila foi responsável por 19.9–31.9% da concentração média de Fe nas águas subterrâneas durante a deposição natural.









Similar content being viewed by others
References
Adhikari D, Zhao Q, Das K, Mejia J, Huang R, Wang X, Poulson SR, Tang Y, Roden EE, Yang Y (2017) Dynamics of ferrihydrite-bound organic carbon during microbial Fe reduction. Geochim Cosmochim Acta 212:221–233
Angerer T, Hagemann SG, Walde DHG (2021) Diagenetic and supergene ore forming processes in the iron formation of the Neoproterozoic Jacadigo Group, Corumbá, Brazil. J S Am Earth Sci 105:102902
Chari NR, Lin Y, Lin YS, Silver WL (2021) Interactive effects of temperature and redox on soil carbon and iron cycling. Soil Biol Biochem 157:108235
Chen C, Dynes JJ, Wang J, Sparks DL (2014) Properties of Fe-organic matter associations via coprecipitation versus adsorption. Environ Sci Technol 48(23):13751–13759
Coward EK, Thompson AT, Plante AF (2017) Iron-mediated mineralogical control of organic matter accumulation in tropical soils. Geoderma 306:206–216
Du Y, Ma T, Deng Y, Shen S, Lu Z (2017) Sources and fate of high levels of ammonium in surface water and shallow groundwater of the Jianghan Plain, Central China. Environ Sci-Proc Imp 19:161–172
Du Y, Deng Y, Ma T, Lu Z, Shen S, Gan Y, Wang Y (2018) Hydrogeochemical evidences for targeting sources of safe groundwater supply in arsenic-affected multi-level aquifer systems. Sci Total Environ 645:1159–1171
Du Y, Deng Y, Ma T, Xu Y, Tao Y, Huang Y, Liu R, Wang Y (2020) Enrichment of geogenic ammonium in Quaternary alluvial-lacustrine aquifer systems: evidence from carbon isotopes and DOM characteristics. Environ Sci Technol 54(10):6104–6114
Egglseder MS, Cruden AR, Tomkins AG, Wilson CJL (2016) Deformation-induced silica redistribution in banded iron formation, Hamersley Province, Australia. Lithos 266–267:87–97
Erban LE, Gorelick SM, Zebker HA, Fendorf S (2013) Release of arsenic to deep groundwater in the Mekong Delta, Vietnam, linked to pumping-induced land subsidence. Proc Natl Acad Sci USA 110(34):13751–13756
Gan Y, Wang Y, Duan Y, Deng Y, Guo X, Ding X (2014) Hydrogeochemistry and arsenic contamination of groundwater in the Jianghan Plain, central China. J Geochem Explor 2014(138):81–93
Guan S, Yang Q, Li Y et al (2022) River flooding response to ENSO-related monsoon precipitation: evidence from late Holocene core sediments in the Jianghan Plain. Palaeogeogr Palaeoclimatol Palaeoecol 589:110834
Gustafsson JP (2011) Visual MINTEQ 3.0 User Guide. KTH, Department of Land and Water Resources, Stockholm
Hou T, Xu RK, Zhao AZ (2007) Interaction between electric double layers of kaolinite and Fe/Al oxides in suspensions. J Colloid Interface Sci 310(2):670
Huang L, Jia X, Zhang G, Thompson A, Chen L (2018) Variations and controls of iron oxides and isotope compositions during paddy soil evolution over a millennial time scale. Chem Geol 476:340–351
Jiao J, Wang Y, Cherry JA, Wang X, Zhi B, Du H, Wen D (2010) Abnormally high ammonium of natural origin in a coastal aquifer-aquitard system in the Pearl River Delta, China. Environ Sci Technol 44:7470–7475
Karakochuk CD, Murphy HM, Whitfield KC, Barr SI, Vercauteren SM, Talukder A, Green TJ (2015) Elevated levels of iron in groundwater in Prey Veng province in Cambodia: a possible factor contributing to high iron stores in women. J Water Health 13(2):575–586
Li J, Wang Y, Zhu C, Xue X, Qian K, Xie X, Wang Y (2020) Hydrogeochemical process controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci Total Environ 730:138877
Liu R (2021) Effects and Mechanism of Fe on organic carbon transformation during the formation of clayey aquitard. PhD Thesis, China University of Geosciences, China
Liu R, Ma T, Qiu W, Du Y, Liu Y (2020a) Effects of Fe oxides on organic carbon variation in the evolution of clayey aquitard and environmental significance. Sci Total Environ 701:134776
Liu R, Ma T, Zhang D, Lin C, Chen J (2020b) Spatial distribution and factors influencing the different forms of ammonium in sediments and pore water of the aquitard along the Tongshun River. China Environ Pollut 266:115212
Liu Y, Ma T, Chen J, Peng Z (2020c) Compaction simulator: a novel device for pressure experiments of subsurface sediments. J Earth Sci 31(5):1045–1050
Liu R, Ma T, Qiu W, Peng Z, Shi C (2020d) The environmental functions and ecological effects of organic carbon in silt. J Earth Sci 31(4):834–844
Loveland PJ, Digby P (1984) The extraction of Fe and Al by 0.1 M pyrophosphate solutions: a comparison of some techniques. J Soil Sci 35(2):243–250
Lovley DR, Holmes DE, Nevin KP (2004) Dissimilatory Fe(III) and Mn(IV) reduction. Adv Microb Physiol 49(2):219
Mehta BC, Shrivastava KK (2012) Iron in groundwater in India and its geochemistry. Mem Ind Soc Anal Geochem 1:209–225
Merrill RD, Shamim AA, Ali H, Labrique AB, Schulze K, Christian P, West KP (2012) High prevalence of anemia with lack of iron deficiency among women in rural Bangladesh: a role for thalassemia and iron in groundwater. Asia Pac J Clin Nutr 21(3):416
Mihajlov I, Mozumder MRH, Bostick BC, Stute M, Mailloux BJ, Knappett PS, Choudhury I, Ahmed KM, Schlosser P, Geen AV (2020) Arsenic contamination of Bangladesh aquifers exacerbated by clay layers. Nat Commun 11(1):1–9
Mondal NC, Singh VS, Puranik SC, Singh VP (2010) Trace element concentration in groundwater of Pesarlanka Island, Krishna Delta. India Environ Monit Assess 163(1–4):215–227
Muehe EM, Scheer L, Daus B, Kappler A (2013) Fate of arsenic during microbial reduction of biogenic versus Abiogenic As-Fe(III)-mineral coprecipitates. Environ Sci Technol 47(15):8297–8307
Parfitt RL, Childs CW (1988) Estimation of forms of Fe and Al: a review and analysis of contrasting soils by dissolution and Mösbauer methods. Aust J Soil Res 26(1):121–144
Schaefer MV, Ying SC, Benner SG, Duan Y, Wang Y, Fendorf S (2016) Aquifer arsenic cycling induced by seasonal hydrologic changes within the Yangtze River Basin. Environ Sci Technol 50(7):3521–3529
Smith RG, Knight R, Fendorf S (2018) Overpumping leads to California groundwater arsenic threat. Nat Commun 9(1):2089
Sun D, Li J, Li H, Liu Q, Zhao S, Huang Y, Wu Q, Xie X (2022) Evolution of groundwater salinity and fluoride in the deep confined aquifers of Cangzhou in the North China plain after the South-to-North Water Diversion Project. Appl Geochem 147:105485
Wagai R, Mayer LM (2007) Sorptive stabilization of organic matter in soils by hydrous iron oxides. Geochim Cosmochim Acta 71(1):25–35
Wagai R, Mayer L, Kitayama K, Shirato Y (2013) Association of organic matter with iron and aluminum across a range of soils determined via selective dissolution techniques coupled with dissolved nitrogen analysis. Biogeochemistry 112(1–3):95–109
Wang X, Di G (2019) Monitoring and analysis of surface deformation in eastern Liaocheng based on PS-In SAR (in Chinese). Bull Surveying Mapping S2:149–153
Wang Y, Jiao JJ, Cherry JA, Lee C (2013) Contribution of the aquitard to the regional groundwater hydrochemistry of the underlying confined aquifer in the Pearl River Delta. China Sci Total Environ 461–462:663–671
Wang Y, Jiao JJ, Zhang K, Zhou Y (2016) Enrichment and mechanisms of heavy metal mobility in a coastal Quaternary groundwater system of the Pearl River Delta, China. Sci Total Environ 545–546:493–502
Weber KA, Achenbach LA, Coates JD (2006) Microorganisms pumping iron: anaerobic microbial iron oxidation and reduction. Nat Rev Microbiol 4(10):752
Xiao C, Ma T, Du Y, Yu H, Shen S (2016) Arsenic releasing characteristics during the compaction of muddy sediments. Environ Sci Processes Impacts 18(1):1297–1304
Xiao C, Ma T, Du Y (2020) Arsenic releasing mechanisms during clayey sediments compaction: an experiment study. J Hydrol 597:125743
Xiong Y, Du Y, Deng Y, Ma T, Li D, Sun X, Liu G, Wang Y (2021) Contrasting sources and fate of nitrogen compounds in different groundwater systems in the central Yangtze River basin. Environ Pollut 290:118119
Xiu W, Yuan W, Polya DA, Guo H, Lloyd JR (2021) A critical review of abiotic and microbially-mediated chemical reduction rates of Fe (III)(oxyhydr) oxides using a reactivity model. Appl Geochem 126:104895
Xue X, Li J, Xie X, Qian K, Wang Y (2019) Impacts of sediment compaction on iodine enrichment in deep aquifers of the North China Plain. Water Res 159:480–489
Zhou J, Du Y, Deng Y, Tao Y, Leng Z, Ma T, Wang Y (2022) Source identification of groundwater phosphorus under different geological settings in the central Yangtze River basin. J Hydrol 612:128169
Acknowledgements
We thank the anonymous reviewers and associate editor for helpful comments that substantially improved the manuscript.
Funding
This study was financially supported by the National Natural Science Foundation of China (Nos. 41630318 and 42007173).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, R., Ma, T., Liu, X. et al. Migration and transformation mechanisms of iron in clayey sediments during compaction: studies using simulation experiments. Hydrogeol J (2024). https://doi.org/10.1007/s10040-024-02783-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10040-024-02783-1