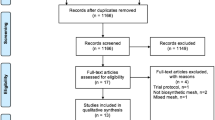
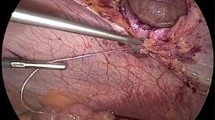
Abstract
Introduction
The objective was to assess the effectiveness and safety of a bioabsorbable mesh at the time of closure of a midline laparotomy for IH prevention.
Materials and Methods
A multicenter, randomized clinical trial including patients undergoing abdominal surgical procedures through a midline laparotomy incision was designed. In the group of mesh (n = 167) the incision was closed using a continuous polydioxanone suture (PDS) plus a bioabsorbable mesh. In the control group (n = 165) a continuous PDS single layer suture was only used. Patients were randomly assigned (1:1) to the two groups. The primary outcome was the incidence of IH at 6, 12 and 24 months. Assessment of IH was done using a CT scan.
Results
At 6 months, the rates of IH were 15.2% and 24.8% in the experimental and control groups, respectively (relative risk [RR] 0.66, 95% confidence interval [CI] 0.38-0.98, P = 0.042). At 12 months, the rate of IH continued to be significantly lower in the experimental group (21.4% vs. 33.1%, P = 0.033), but at 24 months, there were no significant differences between the study groups with a follow-up rate of only 37.5%. The number needed to treat (NNT) was 11 and 9 at 6 and 12 months, respectively.
Conclusion
The bioabsorbable mesh significantly prevented IH during the first year. Not reliable conclusions can be drawn across the second year. This may suggest that the any of the closing technique assessed in this study would have a “palliative” transient effect for preventing IH in the long-term.


Similar content being viewed by others
References
Bosanquet DC, Ansell J, Abdelrahman T, Cornish J, Harries R, Stimpson A, Davies L, Glasbey JC, Frewer KA, Frewer NC, Russell D, Russell I, Torkington J (2015) Systematic review and meta-regression of factors affecting midline incisional hernia rates: analysis of 14,618 patients. PLoS ONE 10:e0138745. https://doi.org/10.1371/journal.pone.0138745
Muysoms FE, Antoniou SA, Bury K, Campanelli G, Conze J, Cuccurullo D, de Beaux AC, Deerenberg EB, East B, Fortelny RH, Gillion JF, Henriksen NA, Israelsson L, Jairam A, Jänes A, Jeekel J, López-Cano M, Miserez M, Morales-Conde S, Sanders DL, Simons MP, Śmietański M, Venclauskas L, Berrevoet F (2015) European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 19(1):1–24. https://doi.org/10.1007/s10029-014-1342-5
Jairam AP, López-Cano M, Garcia-Alamino JM, Pereira JA, Timmermans L, Jeekel J, Lange J, Muysoms F (2020) Prevention of incisional hernia after midline laparotomy with prophylactic mesh reinforcement: a meta-analysis and trial sequential analysis. BJS Open 4(3):357–368. https://doi.org/10.1002/bjs5.50261
Fischer JP, Harris HW, López-Cano M, Hope WW (2019) Hernia prevention: practice patterns and surgeons’ attitudes about abdominal wall closure and the use of prophylactic mesh. Hernia 23(2):329–334. https://doi.org/10.1007/s10029-019-01894-z
López-Cano M, Armengol M, Quiles MT, Biel A, Velasco J, Huguet P, Mestre A, Delgado LM, Gil FX, Arbós MA (2013) Preventive midline laparotomy closure with a new bioabsorbable mesh: an experimental study. J Surg Res 181(1):160–169. https://doi.org/10.1016/j.jss.2012.05.041
López-Cano M, Pereira JA, Lozoya R, Feliu X, Villalobos R, Navarro S, Arbós MA, Armengol-Carrasco M (2014) PREBIOUS trial: a multicenter randomized controlled trial of PREventive midline laparotomy closure with a BIOabsorbable mesh for the prevention of incisional hernia: rationale and design. Contemp Clin Trials 39(2):335–341. https://doi.org/10.1016/j.cct.2014.10.009
Israelsson LA, Millbourn D (2013) Prevention of incisional hernias: how to close a midline incision. Surg Clin North Am 93(5):1027–1040. https://doi.org/10.1016/j.suc.2013.06.009
Berríos-Torres SI, Umscheid CA, Bratzler DW, Leas B, Stone EC, Kelz RR, Reinke CE, Morgan S, Solomkin JS, Mazuski JE, Dellinger EP, Itani KMF, Berbari EF, Segreti J, Parvizi J, Blanchard J, Allen G, Kluytmans JAJW, Donlan R, Schecter WP (2017) Healthcare Infection Control Practices Advisory Committee (2017) Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection. JAMA Surg 152(8):784–791. https://doi.org/10.1001/jamasurg.2017.0904
Kroese LF, Sneiders D, Kleinrensink GJ, Muysoms F, Lange JF (2018) Comparing different modalities for the diagnosis of incisional hernia: a systematic review. Hernia 22(2):229–242. https://doi.org/10.1007/s10029-017-1725-5
Burger JW, Lange JF, Halm JA, KleinrensinK GJ, Jeekel H (2005) Incisional hernia: early complication of abdominal surgery. World J Surg 29(12):1608–1613. https://doi.org/10.1007/s00268-005-7929-3
Cook RJ, Sackett DL (1995) The number needed to treat: a clinically useful measure of treatment effect. BMJ 310(6977):452–454. https://doi.org/10.1136/bmj.310.6977.452
Cruse PJ, Foord R (1980) The epidemiology of wound infection. A 10-year prospective study of wounds. Surg Clin North Am 60(1):27–40. https://doi.org/10.1016/s0039-6109(16)42031-1
Turner MC, Migaly J (2019) Surgical site infection: the clinical and economic impact. Clin Colon Rectal Surg 32(3):157–165. https://doi.org/10.1055/s-0038-1677002
Eke N, Jebbin NJ (2006) Abdominal wound dehiscence: a review. Int Surg 91(5):276–287
López-Cano M, Pereira JA, Armengol-Carrasco M (2013) “Acute postoperative open abdominal wall”: nosological concept and treatment implications. World J Gastrointest Surg 5(12):314–220. https://doi.org/10.4240/wjgs.v5.i12.314
López-Cano M, García-Alamino JM, Antoniou SA, Bennet D, Dietz UA, Ferreira F, Fortelny RH, Hernandez-Granados P, Miserez M, Montgomery A, Morales-Conde S, Muysoms F, Pereira JA, Schwab R, Slater N, Vanlander A, Van Ramshorst GH, Berrevoet F (2018) EHS clinical guidelines on the management of the abdominal wall in the context of the open or burst abdomen. Hernia 22(6):921–939. https://doi.org/10.1007/s10029-018-1818-9
Pereira JA, Pera M, Grande L (2013) Incidence of incisional hernia after open and laparoscopic colorectal cancer resection. Cir Esp 91(1):44–49. https://doi.org/10.1016/j.ciresp.2012.05.004
Fink C, Baumann P, Wente MN, Knebel P, Bruckner T, Ulrich A, Werner J, Büchler MW, Diener MK (2014) Incisional hernia rate 3 years after midline laparotomy. Br J Surg 101(2):51–54. https://doi.org/10.1002/bjs.9364
Diaz R, Quiles MT, Guillem-Marti J, Lopez-Cano M, Huguet P, Ramon-Y-Cajal S, Reventos J, Armengol M, Arbos MA (2011) Apoptosis-like cell death induction and aberrant fibroblast properties in human incisional hernia fascia. Am J Pathol 178(6):2641–2653. https://doi.org/10.1016/j.ajpath.2011.02.044
Glauser PM, Brosi P, Speich B, Käser SA, Heigl A, Rosenberg R, Maurer CA (2019) Prophylactic intraperitoneal onlay mesh following midline laparotomy-long-term results of a randomized controlled trial. World J Surg 43(7):1669–1675. https://doi.org/10.1007/s00268-019-04964-6
Caro-Tarrago A, Olona C, Millán M, Olona M, Espina B, Jorba R (2019) Long-term results of a prospective randomized trial of midline laparotomy closure with onlay mesh. Hernia 23(2):335–340. https://doi.org/10.1007/s10029-019-01891-2
San Miguel C, Melero D, Jiménez E, López P, Robin Á, Blázquez LA, López-Monclús J, González E, Jiménez C, García-Ureña MÁ (2018) Long-term outcomes after prophylactic use of onlay mesh in midline laparotomy. Hernia 22(6):1113–1122. https://doi.org/10.1007/s10029-018-1833-x
Deerenberg EB, Harlaar JJ, Steyerberg EW, Lont HE, van Doorn HC, Heisterkamp J, Wijnhoven BP, Schouten WR, Cense HA, Stockmann HB, Berends FJ, Dijkhuizen FPH, Dwarkasing RS, Jairam AP, van Ramshorst GH, Kleinrensink GJ, Jeekel J, Lange JF (2015) Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet 386(10000):1254–1260. https://doi.org/10.1016/S0140-6736(15)60459-7
McDonald AM, Knight RC, Campbell MK, Entwistle VA, Grant AM, Cook JA, Elbourne DR, Francis D, Garcia J, Roberts I (2006) Snowdon C (2006) What influences recruitment to randomised controlled trials? A review of trials funded by two UK funding agencies. Trials 7:9. https://doi.org/10.1186/1745-6215-7-9
Spaar A, Frey M, Turk A, Karrer W, Puhan MA (2009) Recruitment barriers in a randomized controlled trial from the physicians’ perspective: a postal survey. BMC Med Res Methodol 9:14. https://doi.org/10.1186/1471-2288-9-14
Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH; STOPIT-2 Study Group, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schünemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NK, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KE, Vandvik PO, Coto-Yglesias F, Chrispim PP, Ramsay T (2010) Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA 303(12):1180–1187. https://doi.org/10.1001/jama.2010.310
Freidlin B, Korn EL (2009) Stopping clinical trials early for benefit: impact on estimation. Clin Trials 6(2):119–125. https://doi.org/10.1177/1740774509102310
Korn EL, Freidlin B, Mooney M (2009) Stopping or reporting early for positive results in randomized clinical trials: the National Cancer Institute Cooperative Group experience from 1990 to 2005. J Clin Oncol 27(10):1712–1721. https://doi.org/10.1200/JCO.2008.19.5339
Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, Mulla S, Lamontagne F, Bassler D, Vera C, Alshurafa M, Katsios CM, Zhou Q, Cukierman-Yaffe T, Gangji A, Mills EJ, Walter SD, Cook DJ, Schünemann HJ, Altman DG, Guyatt GH (2012) Potential impact on estimated treatment effects of information lost to follow-up in randomised controlled trials (LOST-IT): systematic review. BMJ 344:e2809. https://doi.org/10.1136/bmj.e2809
Acknowledgements
We thank Marta Pulido, MD, PhD, for editing the manuscript and editorial assistance.
Funding
The trial is an investigator-initiated study supported in part by W.L. Gore and Associates, S.L. (PR [AG] 220/2013) without scientific influence.
Author information
Authors and Affiliations
Contributions
Silvia Valverde contributed to collected data, analyze data, interpreted the results, revised the manuscript for intellectual content, and approved the final draft. Maria Antonia Arbós participated in the collection of data, search and review of the literature, analysis of results, review of the manuscript, and approved the final draft. Maria Teresa Quiles participated in the collection of data, search and review of the literature, analysis of results, review of the manuscript, and approved the final draft. Eloy Espín contributed to search of the literature, interpretation of results and approval of the final draft. Jose Luis Sánchez contributed to search of the literature, interpretation of results and approval of the final draft. Victor Rodrigues contributed to collection data, search of the literature, interpretation of results and approval of the final draft. Jose Antonio Pereira contributed to collection data, interpretation of results and approval of the final draft. Rafael Villalobos contributed to collection data, interpretation of results and approval of the final draft. Josep M García-Alamino participated in the collection of data, search and review of the literature, analysis of results, review of the manuscript, and approved the final draft. Manuel Armengol contributed to search of the literature, interpretation of results and approval of the final draft. Manuel López-Cano was the principal investigator and the trial coordinator, designed the study, collected data, contributed to statistical analysis, and drafted the manuscript. He was also responsible for editorial decisions including the selection of the journal.
Corresponding author
Ethics declarations
Conflict of interests
López-Cano has received honoraria for consultancy, lectures, support for travels and participation in review activities from BD-Bard, Medtronic and Gore. S Valverde, MA Arbós, MT Quiles, E Espin, JL Sanchez-García, V Rodrigues, JA Pereira, R Villalobos, JM García-Alamino and M Armengol have no conflicts of interest or financial ties to disclose.
Ethical approval
The study was approved by the Ethics Committee of Clinical Research of Hospital Universitari Vall d’Hebron (PR [AG] 220/2013).
Human and animal rights
This article does not contain any study with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file3 (MP4 8656 kb)
Rights and permissions
About this article
Cite this article
Valverde, S., Arbós, M.A., Quiles, M.T. et al. Use of a bioabsorbable mesh in midline laparotomy closure to prevent incisional hernia: randomized controlled trial. Hernia 26, 1231–1239 (2022). https://doi.org/10.1007/s10029-021-02435-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-021-02435-3