Abstract
Purpose
More than 350,000 ventral hernia repairs are performed in the U.S. each year. However, long-term quality of life of patients living with hernia repair is less known. Follow-up using patient-reported outcomes (measures) is an important representation of the patient experience and can inform quality improvements of hernia treatments. This study aims to understand the patients’ experience after ventral hernia repair, to enhance quality of care and long-term hernia treatment outcomes.
Methods
To better understand long-term outcomes of ventral hernia repair and to enhance an existing PRO tool, two rounds of semi-structured interviews and focus groups were conducted. In total, 22 patients who had ventral hernia repair were enrolled. The patient perspectives obtained were grouped into themes to inform the further development of the PRO tool. Data were transcribed and analyzed using atlas.ti and Microsoft Word.
Results
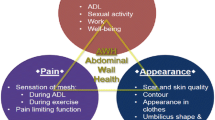
Ten major themes were identified in this analysis. Patients’ quality of life was impacted by hernia repairs and hernia recurrences, including chronic pain, effects on daily activities and social relationships, and the challenge in finding new treatments. The lack of provider–patient communication and patient understanding of hernia repairs highlighted the need for providing patients with more comprehensive information regarding repair options and outcomes prior to surgery.
Conclusion
PRO assessments and meaningful communications between the physician and the patient can provide a comprehensive benefit–risk assessment prior to surgery, and may also improve patient understanding of what to expect during recovery from surgery.
Similar content being viewed by others
References
Poulose BK, Shelton J, Phillips S, Moore D, Nealon W, Penson D, Beck W, Holzman MD (2012) Epidemiology and cost of ventral hernia repair: making the case for hernia research. Hernia 16(2):179–183. https://doi.org/10.1007/s10029-011-0879-9
Baylon K, Rodriguez-Camarillo P, Elias-Zuniga A, Diaz-Elizondo JA, Gilkerson R, Lozano K (2017) Past, present and future of surgical meshes: a review. Membranes. https://doi.org/10.3390/membranes7030047
Bleier JIS, Resnick AS (2009) Complications of incisional hernia repair. Semin Colon Rect Surg 20(3):125–130. https://doi.org/10.1053/j.scrs.2009.06.004
Patient Engagement Advisory Committee (2017) FDA executive summary: documents for patient engagement in medical device clinical trials meeting
U.S. Food and Drug Administration (2009) Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Federal Register
Baucom RB, Ousley J, Feurer ID, Beveridge GB, Pierce RA, Holzman MD, Sharp KW, Poulose BK (2016) Patient reported outcomes after incisional hernia repair-establishing the ventral hernia recurrence inventory. Am J Surg 212(1):81–88. https://doi.org/10.1016/j.amjsurg.2015.06.007
Americas Hernia Society Quality Collaborative (2016) FAQs. https://www.ahsqc.org/faqs. Accessed Sep 2016
Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, Petrillo J, Powers J, Sethi S, Group E-PS (2010) Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health 13(8):965–975. https://doi.org/10.1111/j.1524-4733.2010.00772.x
Rothman M, Burke L, Erickson P, Leidy NK, Patrick DL, Petrie CD (2009) Use of existing patient-reported outcome (PRO) instruments and their modification: the ISPOR good research practices for evaluating and documenting content validity for the use of existing instruments and their modification PRO task force report. Value Health 12(8):1075–1083. https://doi.org/10.1111/j.1524-4733.2009.00603.x
Gill P, Stewart K, Treasure E, Chadwick B (2008) Methods of data collection in qualitative research: interviews and focus groups. Br Dent J 204(6):291–295. https://doi.org/10.1038/bdj.2008.192
Patrick DLBL, Gwaltney CJ et al (2011) Content validity—establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1—eliciting concepts for a new PRO instrument. Value Health 14:967–977
U.S. Food and Drug Administration (2018) MedSun: medical product safety network. https://www.fda.gov/MedicalDevices/Safety/MedSunMedicalProductSafetyNetwork/default.htm. Accessed 5 Feb 2018
Friese S (2017) ATLAS.ti 8 Windows user manual. Scientific Software Development GmbH, Berlin, Germany
Ha JF (2010) Doctor-patient communication: a review. Ochsner J 10(1):38–43
Friedman AJ, Cosby R, Boyko S, Hatton-Bauer J, Turnbull G (2011) Effective teaching strategies and methods of delivery for patient education: a systematic review and practice guideline recommendations. J Cancer Educ 26(1):12–21. https://doi.org/10.1007/s13187-010-0183-x
Stern C, Lockwood C (2005) Knowledge retention from preoperative patient information. Int J Evid Based Healthc 3(3):45–63. https://doi.org/10.1111/j.1479-6988.2005.00021.x
Rotenstein LS, Huckman RS, Wagle NW (2017) Making patients and doctors happier—the potential of patient-reported outcomes. N Engl J Med 377(14):1309–1312. https://doi.org/10.1056/NEJMp1707537
Acknowledgements
The authors would like to acknowledge Dr. Benjamin Poulose and Dr. Michael Rosen of the Americas Hernia Society Quality Collaborative for their significant contributions to this work.
Funding
No funds or support has been received for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflict of interest.
Ethical approval
Experiments comply with the current laws of the country in which they were performed.
Human and animal rights
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent for participation was obtained before the interviews began.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendices
Appendix 1: HerQLes—Hernia-related quality-of-life assessment tool

Appendix 2: Interview and Focus Group Semi-Structured Script
General questions
-
1.
Can you tell me a little bit about how you got your hernia?
-
2.
What was living with hernia like before surgery?
-
3.
What type of treatment did you get? (How did you feel about the procedure?)
-
4.
How did you physically feel after the procedure until now?
-
5.
How long after the procedure did you notice symptoms? How did they affect you (how intense and frequent were they)?
-
6.
What kind of (other) symptoms did you experience?
-
7.
What made/makes the symptoms better?
-
8.
Did you experience any changes in your symptoms as time went on? (The trend of symptoms)
-
9.
Was that what you expected for recovery? Were any new or unexpected?
-
10.
What was making the decision for taking the treatment like?
Did you have any concerns? (worried about anything/potential side effects)
PRO survey questions
Instructions: Now we are going to look at this survey. This survey will be used to collect data from people who had a hernia surgery, and to track their outcomes over time. So having had a surgery yourself, can we have your thoughts on what would make sense in a survey? So, what would make a ‘perfect’ survey?
-
11.
What are some of the thoughts that came to mind when you were doing this survey?
-
12.
Are there other items or questions that should have been in the survey?
-
13.
Which questions are the least important and could probably be taken off the list?
-
14.
Which questions are the most important to you?
-
15.
Any phrasing or wording of the questions that should be written in a different way?
-
16.
How was the length of the survey?
-
17.
What instruction did you receive when doing the survey? (how to do it, and how frequent you will have to do it?)
Rights and permissions
About this article
Cite this article
Lee, TH.J., Ulisney, K.L., Choudhuri, A.K. et al. Understanding the patient perspective after ventral hernia repair. Hernia 23, 995–1001 (2019). https://doi.org/10.1007/s10029-019-02015-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-019-02015-6