Abstract
Purpose
Biologic meshes have unique physical properties as a result of manufacturing techniques such as decellularization, crosslinking, and sterilization. The purpose of this study is to directly compare the biocompatibility profiles of five different biologic meshes, AlloDerm® (non-crosslinked human dermal matrix), PeriGuard® (crosslinked bovine pericardium), Permacol® (crosslinked porcine dermal matrix), Strattice® (non-crosslinked porcine dermal matrix), and Veritas® (non-crosslinked bovine pericardium), using a porcine model of ventral hernia repair.
Methods
Full-thickness fascial defects were created in 20 Yucatan minipigs and repaired with the retromuscular placement of biologic mesh 3 weeks later. Animals were euthanized at 1 month and the repair sites were subjected to tensile testing and histologic analysis. Samples of unimplanted (de novo) meshes and native porcine abdominal wall were also analyzed for their mechanical properties.
Results
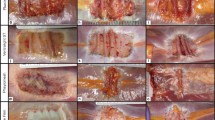
There were no significant differences in the biomechanical characteristics between any of the mesh-repaired sites at 1 month postimplantation or between the native porcine abdominal wall without implanted mesh and the mesh-repaired sites (P > 0.05 for all comparisons). Histologically, non-crosslinked materials exhibited greater cellular infiltration, extracellular matrix (ECM) deposition, and neovascularization compared to crosslinked meshes.
Conclusions
While crosslinking differentiates biologic meshes with regard to cellular infiltration, ECM deposition, scaffold degradation, and neovascularization, the integrity and strength of the repair site at 1 month is not significantly impacted by crosslinking or by the de novo strength/stiffness of the mesh.






Similar content being viewed by others
References
Burger JW, Luijendijk RW, Hop WCJ, Halm JA, Verdaasdonk EGG, Jeekel J (2004) Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg 240(4):578–585
Rosen M, Brody F, Ponsky J, Walsh RM, Rosenblatt S, Duperier F, Fanning A, Siperstein A (2003) Recurrence after laparoscopic ventral hernia repair: a five-year experience. Surg Endosc 17:123–128
Maurice SM, Skeete DA (2009) Use of human acellular dermal matrix for abdominal wall reconstructions. Am J Surg 197:35–42
Kaufman Z, Engelberg M, Zager M (1981) Fecal fistula: a late complication of Marlex mesh repair. Dis Colon Rectum 24(7):543–544
Leber GE, Garb JL, Alexander AI, Reed WP (1998) Long-term complications associated with prosthetic repair of incisional hernias. Arch Surg 133:378–382
Klinge U, Klosterhalfen B, Müller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165:665–673
Diaz JJ Jr, Conquest AM, Ferzoco SJ, Vargo D, Miller P, Wu YC, Donahue R (2009) Multi-institutional experience using human acellular dermal matrix for ventral hernia repair in a compromised surgical field. Arch Surg 144(3):209–215
Milburn ML, Holton LH, Chung TL, Li EN, Bochicchio GV, Goldberg NH, Silverman RP (2008) Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri-operative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect 9(4):433–442
Carbonell AM, Matthews BD, Dréau D, Foster M, Austin CE, Kercher KW, Sing RF, Heniford BT (2005) The susceptibility of prosthetic biomaterials to infection. Surg Endosc 19:430–435
Petter-Puchner AH, Fortelny RH, Walder N, Mittermayr R, Ohlinger W, van Griensven M, Redl H (2008) Adverse effects associated with the use of porcine cross-linked collagen implants in an experimental model of incisional hernia repair. J Surg Res 145:105–110
Hiles M, Record Ritchie RD, Altizer AM (2009) Are biologic grafts effective for hernia repair? A systematic review of the literature. Surg Innov 16:26–37
Candage R, Jones K, Luchette FA, Sinacore JM, Vandevender D, Reed RL 2nd (2008) Use of human acellular dermal matrix for hernia repair: friend or foe? Surgery 144(4):703–711
Franklin ME Jr, Gonzalez JJ Jr, Michaelson RP, Glass JL, Chock DA (2002) Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia 6:171–174
Duan X, Sheardown H (2005) Crosslinking of collagen with dendrimers. J Biomed Mater Res A 75(3):510–518
Abhinav K, Shaaban M, Raymond T, Oke T, Gullan R, Montgomery ACV (2009) Primary reconstruction of pelvic floor defects following sacrectomy using Permacol graft. Eur J Surg Oncol 35:439–443
Hammond TM, Chin-Aleong J, Navsaria H, Williams NS (2008) Human in vivo cellular response to a cross-linked acellular collagen implant. Br J Surg 95:438–446
Pierce LM, Rao A, Baumann SS, Glassberg JE, Kuehl TJ, Muir TW (2009) Long-term histologic response to synthetic and biologic graft materials implanted in the vagina and abdomen of a rabbit model. Am J Obstet Gynecol 200:546.e1–546.e8
Committee on Care and Use of Laboratory Animals (1996) US Department of Health and Human Services, Public Health Service, National Institutes of Health, Bethesda, MD
Jenkins ED, Melman L, Desai S, Brown SR, Frisella MM, Deeken CR, Matthews BD (2010) Evaluation of intraperitoneal placement of absorbable and nonabsorbable barrier coated mesh secured with fibrin sealant in a New Zealand white rabbit model. Surg Endosc [Epub ahead of print]
Valentin JE, Badylak JS, McCabe GP, Badylak SF (2006) Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am 88:2673–2686
Acknowledgments
This research was supported by a grant from Synovis Life Sciences, St. Paul, MN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Melman, L., Jenkins, E.D., Hamilton, N.A. et al. Early biocompatibility of crosslinked and non-crosslinked biologic meshes in a porcine model of ventral hernia repair. Hernia 15, 157–164 (2011). https://doi.org/10.1007/s10029-010-0770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-010-0770-0