Abstract
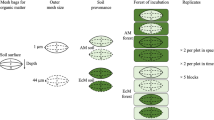
Soil respiration is the dominant pathway by which terrestrial carbon enters the atmosphere. Many abiotic and biotic processes can influence soil respiration, including soil microbial community composition. Mycorrhizal fungi are a particularly important microbial group because they are known to influence soil chemistry and nutrient cycling, and, because the type of mycorrhizal fungi in an ecosystem can be assessed based on the plant species present, they may be easier than other soil microbes to incorporate into ecosystem models. We tested how the type of mycorrhizal fungi—arbuscular (AM) or ectomycorrhizal (ECM) fungi—associated with the dominant tree species in a mixed hardwood forest was related to soil respiration rate. We measured soil respiration, root biomass, and surface area, and soil chemical and physical characteristics during the growing season in plots dominated by ECM-associated trees, AM-associated trees, and mixtures with both. We found rates of soil respiration that were 29% and 32% higher in AM plots than in ECM and mixed plots, respectively. These differences are likely explained by the slightly higher nitrogen concentrations and deeper organic horizons in soil within AM plots compared with soil in ECM and mixed plots. Our results highlight the importance of considering mycorrhizal associations of dominant vegetation as predictors of carbon cycling processes.



Similar content being viewed by others
References
Allison SD, LeBauer DS, Ofrecio MR, Reyes R, Ta A-M, Tran TM. 2009. Low levels of nitrogen addition stimulate decomposition by boreal forest fungi. Soil Biol Biochem 41:293–302.
Bae K, Fahey TJ, Yanai RD, Fisk M. 2015. Soil nitrogen availability affects belowground carbon allocation and soil respiration in northern hardwood forests of New Hampshire. Ecosystems 18:1179–91.
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P. 2004. Chronic nitrogen additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manag 196:43–56.
Brundrett MC. 2002. Coevolution of roots and mycorrhiza of land plants. New Phytol 154:275–304.
Brzostek ER, Dragoni D, Brown ZA, Phillips RP. 2015. Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol 206:1274–82.
Burton AJ, Pregitzer KS, Crawford JN, Zogg GP, Zak DR. 2004. Simulated chronic NO3−deposition reduces soil respiration in northern hardwood forests. Glob Chang Biol 10:1080–91.
Büttner V, Leuschner C. 1994. Spatial and temporal patterns of fine root abundance in a mixed oak-beech forest. For Ecol Manag 70:11–21.
Carney KM, Matson PA. 2005. Plant communities, soil microorganisms, and soil carbon cycling: does altering the world belowground matter to ecosystem functioning? Ecosystems 8:928–40.
Cheeke TE, Phillips RP, Brzostek ER, Rosling A, Bever JD, Fransson P. 2016. Dominant mycorrhizal association of trees alters carbon and nutrient cycling by selecting for microbial groups with distinct enzyme function. New Phytol 214:432–42.
Cornelissen J, Aerts R, Cerabolini B, Werger M, van der Heijden M. 2001. Carbon cycling traits of plant species are linked with mycorrhizal strategy. Oecologia 129:611–19.
Courty PE, Buée M, Diedhiou AG, Frey-Klett P, Le Tacon F, Rineau F, Turpault MP, Uroz S, Garbaye J. 2010. The role of ectomycorrhizal communities in forest ecosystem processes: new perspectives and emerging concepts. Soil Biol Biochem 42:679–98.
Craig ME, Turner BL, Liang C, Clay K, Johnson DJ, Phillips RP. 2018. Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Glob Chang Biol 24:3317–30.
Creelman C, Nickerson N, Risk D. 2013. Quantifying lateral diffusion error in soil carbon dioxide respiration estimates using numerical modeling. Soil Sci Soc Am J 77:699–708.
Davidson EA, Savage K, Verchot LV, Navarro R. 2002. Minimizing artifacts and biases in chamber-based measurements of soil respiration. Agric For Meteorol 113:21–37.
Fernandez C, Kennedy PG. 2015. Revisiting the ‘Gadgil effect’: do interguild fungal interactions control carbon cycling in forest soils? New Phytol:1–17.
Fisk M, Santangelo S, Minick K. 2015. Carbon mineralization is promoted by phosphorus and reduced by nitrogen addition in the organic horizon of northern hardwood forests. Soil Biol Biochem 81:212–18.
Frey SD, Knorr M, Parrent JL, Simpson RT. 2004. Chronic nitrogen enrichment affects the structure and function of the soil microbial community in temperate hardwood and pine forests. For Ecol Manag 196:159–71.
Gadgil R, Gadgil PD. 1971. Mycorrhiza and litter decomposition. Nature 233:133.
Giasson M-A, Ellison AM, Bowden RD, Crill PM, Davidson EA, Drake JE, Frey SD, Hadley JL, Lavine M, Melillo JM, Munger JW, Nadelhoffer KJ, Nicoll L, Ollinger SV, Savage KE, Steudler PA, Tang J, Varner RK, Wofsy SC, Foster DR, Finzi AC. 2013. Soil respiration in a northeastern US temperate forest: a 22-year synthesis. Ecosphere 4:1–28.
Gui H, Hyde K, Xu J, Mortimer P. 2017. Arbuscular mycorrhiza enhance the rate of litter decomposition while inhibiting soil microbial community development. Sci Rep:1–11.
Herman DJ, Firestone MK, Nuccio E, Hodge A. 2012. Interactions between an arbuscular mycorrhizal fungus and a soil microbial community mediating litter decomposition. FEMS Microbiol Ecol 80:236–47.
Hodge A. 2004. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24.
Huang N, Gu L, Niu Z. 2014. Estimating soil respiration using spatial data products: a case study in a deciduous broadleaf forest in the Midwest USA. J Geophys Res 119:6393–408.
Janssens IA, Dieleman W, Luyssaert S, Subke J-A, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze E-D, Tang J, Law BE. 2010. Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315.
Keeler BL, Hobbie SE, Kellogg LE. 2009. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15.
Koide RT, Fernandez CW, Peoples MS. 2011. Can ectomycorrhizal colonization of Pinus resinosa roots affect their decomposition? New Phytol 191:508–14.
Kong D, Wang J, Wu H, Valverde-Barrantes OJ, Wang R, Zeng H, Kardol P, Zhang H, Feng Y. 2019. Nonlinearity of root trait relationships and the root economics spectrum. Nat Commun 10:2203.
Langley JA, Chapman SK, Hungate BA. 2006. Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol Lett 9:955–9.
Lin G, McCormack ML, Ma C, Guo D. 2017. Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytol 213:1440–51.
Liu C, Xiang W, Zou L, Lei P, Zeng Y, Ouyang S, Deng X, Fang X, Liu Z, Peng C. 2019. Variation in the functional traits of fine roots is linked to phylogenetics in the common tree species of Chinese subtropical forests. Plant Soil 436:347–64.
Maestre FT, Cortina J. 2003. Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Appl Soil Ecol 23:199–209.
Maier CA, Kress LW. 2000. Soil CO, evolution and root respiration in 11 year-old loblolly pine (Pinus Weda) plantations as affected by moisture and nutrient availability. For Res 30:347–59.
Martin JG, Bolstad PV, Ryu S-R, Chen J. 2009. Modeling soil respiration based on carbon, nitrogen, and root mass across diverse Great Lake forests. Agric For Meteorol 149:1722–9.
Matthes J, Lang A, Jevon F, Russell S. 2018. Tree stress and mortality from emerald ash borer does not systematically alter short-term soil carbon flux in a mixed northeastern U.S. forest. Forests 9:37.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Monson RK, Lipson DL, Burns SP, Turnipseed AA, Delany AC, Williams MW, Schmidt SK. 2006. Winter forest soil respiration controlled by climate and microbial community composition. Nature 439:711–14.
Nuccio EE, Hodge A, Pett-Ridge J, Herman DJ, Weber PK, Firestone MK. 2013. An arbuscular mycorrhizal fungus significantly modifies the soil bacterial community and nitrogen cycling during litter decomposition. Environ Microbiol 15:1870–81.
Paterson E, Sim A, Davidson J, Daniell TJ. 2016. Arbuscular mycorrhizal hyphae promote priming of native soil organic matter mineralisation. Plant Soil 408:243–54.
Phillips RP, Fahey TJ. 2006. Tree species and mycorrhizal associations influence the magnitude of rhizosphere effects. Ecology 87:1302–13.
Phillips RP, Fahey TJ. 2007. Fertilization effects on fineroot biomass, rhizosphere microbes and respiratory fluxes in hardwood forest soils. New Phytol 176:655–64.
Phillips RP, Brzostek E, Midgley MG. 2013. The mycorrhizal-associated nutrient economy: a new framework for predicting carbon-nutrient couplings in temperate forests. New Phytol 199:41–51.
Pierret A, Gonkhamdee S, Jourdan C, Maeght J-L. 2013. IJ_Rhizo: an open-source software to measure scanned images of root samples. Plant Soil 373:531–9.
Pinheiro J, Bates D, DebRoy S, Sarkar D. 2019. nlme: linear and nonlinear mixed effects models. https://cran.r-project.org/package=nlme
R Core Team. 2018. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing https://www.R-project.org/
Raich JW, Potter CS, Bhagawati D. 2002. Interannual variability in global soil respiration, 1980–94. Glob Chang Biol 8:800–12.
Read DJ, Perez-Moreno J. 2003. Mycorrhizas and nutrient cycling in ecosystems—a journey towards relevance? New Phytol 157:475–92.
Rillig MC, Wright SF, Nichols KA, Schmidt WF, Torn MS. 2001. Large contribution of arbuscular mycorrhizal fungi to soil carbon pools in tropical forest soils. Plant Soil 233:167–77.
Savage KE, Davidson EA. 2001. Interannual variation of soil respiration in two New England forests. Glob Biogeochem Cycles 15:337–50.
Schlesinger W, Andrews J. 2000. Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20.
Schwarz PA, Fahey TJ, McCulloch CE. 2003. Factors controlling spatial variation of tree species abundance in a forested landscape. Ecology 84:1862–78.
Sinsabaugh RL, Carreiro MM, Repert DA. 2002. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 60:1–24.
Smith SE, Read DJ. 2010. Mycorrhizal symbiosis. Cambridge: Academic Press.
Sommerville D, Bradley R, Mailly D. 2004. Leaf litter quality and decomposition rates of yellow birch and sugar maple seedlings grown in mono-culture and mixed-culture pots at three soil fertility levels. Trees 18:608–13.
Soudzilovskaia NA, Heijden MGAVD, Cornelissen JHC, Mikhail I. 2015. Quantitative assessment of the differential impacts of arbuscular and ectomycorrhiza on soil carbon cycling. New Phytol 208:280–93.
Stoyan H, De-Polli H, Böhm S, Robertson GP, Paul EA. 2000. Spatial heterogeneity of soil respiration and related properties at the plant scale. Plant Soil 222:203–14.
Talbot JM, Allison SD, Treseder KK. 2008. Decomposers in disguise: mycorrhizal fungi as regulators of soil C dynamics in ecosystems under global change. Funct Ecol 22:955–63.
Tang J, Baldocchi DD. 2005. Spatial–temporal variation in soil respiration in an oak–grass savanna ecosystem in California and its partitioning into autotrophic and heterotrophic components. Biogeochemistry 73:183–207.
Taylor MK, Lankau R, Wurzburger N. 2016. Mycorrhizal associations of trees have different indirect effects on organic matter decomposition. J Ecol 104:1576–84.
USDA Forest Service. 1996. Hubbard Brook Ecosystem Study. Site Description and Research Activities. Northeastern Forest Experiment Station USDA Forest Service, NE-INF-96-96R Second Edition.
Valverde-Barrantes OJ, Smemo KA, Feinstein LM, Kershner MW, Blackwood CB. 2018. Patterns in spatial distribution and root trait syndromes for ecto and arbuscular mycorrhizal temperate trees in a mixed broadleaf forest. Oecologia 186:731–41.
van der Heijden MGA, Martin FM, Selosse M-AA, Sanders IR. 2015. Mycorrhizal ecology and evolution: the past, the present, and the future. New Phytol 205:1406–23.
Vargas R, Baldocchi DD, Querejeta JI, Curtis PS, Hasselquist NJ, Janssens IA, Allen MF, Montagnani L. 2010. Ecosystem CO2 fluxes of arbuscular and ectomycorrhizal dominated vegetation types are differentially influenced by precipitation and temperature. New Phytol 185:226–36.
Wang X, Wang C. 2018. Mycorrhizal associations differentiate soil respiration in five temperate monocultures in Northeast China. For Ecol Manag 430:78–85.
Wang WJ, Dalal RC, Moody PW, Smith CJ. 2003. Relationships of soil respiration to microbial biomass, substrate availability and clay content. Soil Biol Biochem 35:273–84.
Wang C, Ma Y, Trogisch S, Huang Y, Geng Y, Scherer-Lorenzen M, He J-S. 2017. Soil respiration is driven by fine root biomass along a forest chronosequence in subtropical China. J Plant Ecol 10:36–46.
Wei H, Chen X, Xiao G, Guenet B, Vicca S, Shen W. 2015. Are variations in heterotrophic soil respiration related to changes in substrate availability and microbial biomass carbon in the subtropical forests? Sci Rep 5:18370.
Wurzburger N, Brookshire ENJ. 2017. Experimental evidence that mycorrhizal nitrogen strategies affect soil carbon. Ecology 98:1491–7.
Yanai RD, Fisk MC, Fahey TJ, Cleavitt NL, Park BB. 2008. Identifying roots of northern hardwood species: patterns with diameter and depth. Can J For Res 38:2862–9.
Yang K, Zhu J, Zhang M, Yan Q, Sun OJ. 2010. Soil microbial biomass carbon and nitrogen in forest ecosystems of Northeast China: a comparison between natural secondary forest and larch plantation. J Plant Ecol 3:175–82.
Zhou L, Zhou X, Zhang B, Lu M, Luo Y, Liu L, Li B. 2014. Different responses of soil respiration and its components to nitrogen addition among biomes: a meta-analysis. Glob Chang Biol 20:2332–43.
Zhu K, McCormack ML, Lankau RA, Egan JF, Wurzburger N. 2018. Association of ectomycorrhizal trees with high carbon-to-nitrogen ratio soils across temperate forests is driven by smaller nitrogen not larger carbon stocks. Clemmensen K, editor. J Ecol 106:524–35.
Acknowledgements
We thank Maanav Jalan and Alex Salazar for field assistance, and Brianna Hibner, Lacey Berg, Catherine D’Hennezel, Carolina Jimenez, Annalise Michaelson, and Sage Wentzell-Brehme for laboratory assistance. We appreciate the support of Geoff Wilson and Natalie Cleavitt in site selection. The Hubbard Brook Experimental Forest is administered by the US Department of Agriculture Forest Service, Northern Forest Research Station, Newtown Square, PA. The Hubbard Brook Long-Term Ecological Research site is funded by NSF award 1637685. Funding for this project was provided by Dartmouth College and the Wellesley College Office of the Provost.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lang, A.K., Jevon, F.V., Ayres, M.P. et al. Higher Soil Respiration Rate Beneath Arbuscular Mycorrhizal Trees in a Northern Hardwood Forest is Driven by Associated Soil Properties. Ecosystems 23, 1243–1253 (2020). https://doi.org/10.1007/s10021-019-00466-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00466-7