Abstract
Tree mortality from insect infestations can significantly reduce carbon storage in forest soils. In subarctic birch forests (Betula pubescens), ecosystem C cycling is largely affected by recurrent outbreaks of defoliating geometrid moths (Epirrita autumnata, Operophtera brumata). Here, we show that soil C stocks in birch forests across Fennoscandia did not change up to 8 years after moth outbreaks. We found that a decrease in woody fine roots was accompanied by a lower soil CO2 efflux rate and a higher soil N availability following moth outbreaks. We suggest that a high N availability and less ectomycorrhiza likely contributed to lowered heterotrophic respiration and soil enzymatic activity. Based on proxies for decomposition (heterotrophic respiration, phenol oxidase potential activity), we conclude that a decrease in decomposition is a prime cause why soil C stocks of mountain birch forest ecosystems have not changed after moth outbreaks. Compared to disturbed temperate and boreal forests, a CO2-related positive feedback of forest disturbance on climate change might therefore be smaller in subarctic regions.
Similar content being viewed by others
Highlights
-
Soil C stocks in subarctic birch forests did not change after moth outbreaks.
-
A high N availability lowered enzymatic activity and CO2 efflux from decomposition.
-
Positive feedback on climate change might be smaller compared to other ecosystems.
Introduction
Northern forest soils store large amounts of carbon (C) and act thereby as a globally important sink for atmospheric CO2 (Pan and others 2011; Bradshaw and Warkentin 2015). The size of soil C stocks depends largely on the balance between organic matter (OM) input to the soil (C gain) and its decomposition by heterotrophs (C loss). Pulses of tree mortality from insect infestations and other disturbances (for example, windthrow, fire) affect this balance. Across disturbed temperate and boreal forests, soil C loss through decomposition seems to predominate—resulting in reduced soil C stocks following disturbance events (Thom and Seidl 2015; Zhang and others 2015). If photosynthetic C fixation cannot compensate for post-disturbance C losses, this may cause a positive feedback on rising atmospheric CO2 concentrations and thus on climate change (Bonan 2008; Kurz and others 2008). In contrast to temperate and boreal forests (Thom and Seidl 2015; Zhang and others 2015), disturbance effects on soil C stocks and on mechanisms underlying decomposition processes remain uncertain for subarctic forests. However, because high-latitude regions are particularly vulnerable to climate warming (IPCC 2014), this information is key to assess additional disturbance-mediated feedback loops to the climate system (Thom and others 2017).
Subarctic deciduous forests cover close to 30,000 km2 of northern Fennoscandia, and the majority of the area is dominated by mountain birch (Betula pubescens var. pumila) (Normander and others 2009; Kuuluvainen and others 2017). The ecosystem C cycle in mountain birch forests is strongly affected by recurrent and abrupt outbreaks of geometrid moths (Epirrita autumnata, Operophtera brumata) (Heliasz and others 2011; Olsson and others 2017). Severe moth outbreaks can cause defoliation over thousands of square kilometres within a few years. In the 1960s, about 5000 km2 were reported to be defoliated in northern Finland (Tenow and Bylund 2000), and during the 2000s, about 10,000 km2 were estimated to be defoliated in northern Fennoscandia (Jepsen and others 2009a). Repeated defoliation events (over the course of several years) exceed the stress tolerance of trees and cause extensive forest mortality (Jepsen and others 2013). A range expansion of moth outbreaks has been observed in recent decades (Jepsen and others 2008). This expansion is possibly caused by more favourable winter temperatures, enhancing the survival of overwintering eggs (Virtanen and others 1998; Neuvonen and others 1999), and by an increased phenological match between egg hatch and birch bud burst (Jepsen and others 2009b). Warmer conditions under future climates could therefore intensify moth outbreaks in subarctic forests—comparable to intensified bark beetle outbreaks in boreal forests (Ramsfield and others 2016).
Defoliation by moth outbreaks is known to reduce C fixation by plants (Heliasz and others 2011; Dahl and others 2017; Olsson and others 2017). A consequent decrease in belowground translocation of C may affect tree root activity (for example, respiration, exudation) and biomass (Högberg and others 2001; Scott-Denton and others 2006). However, it can also influence soil microbial community structure (Kaiser and others 2010; Pena and others 2010) and soil nitrogen (N) cycling, enzymatic activity and heterotrophic respiration (Weintraub and others 2007; Kaiser and others 2011; Brzostek and others 2015). In accordance, subarctic birch stands affected by moth outbreaks featured a reduction in fine roots, ectomycorrhizal fungi and soil CO2 efflux (Saravesi and others 2015; Parker and others 2017) and an increased N availability, for example, due to frass deposition or reduced N uptake by vegetation (Kosola and others 2001; Kaukonen and others 2013; Parker and others 2017). Although increased N supply can have both accelerating and decelerating effects on OM decomposition (Averill and Waring 2018), decomposition of more recalcitrant OM (for example, in humus layers) could be demonstrated to decrease under increasing N (Craine and others 2007; Janssens and others 2010; Ramirez and others 2012). In high-latitude forests, for example, OM degrading enzymes released from ectomycorrhizal fungi (Bödeker and others 2014) and soil C turnover (Baskaran and others 2017) were highly sensitive to N availability. Thus, OM decomposition might be reduced following moth defoliation accompanied by increases in N availability. In contrast to other forest ecosystems (Thom and Seidl 2015; Zhang and others 2015), post-disturbance soil C losses and changes in soil C stocks might therefore be small or even negligible in disturbed subarctic birch forest ecosystems. Although previous findings point towards reduced decomposition rates following moth outbreaks, and possible reasons for slowed biochemical dynamics have been speculated on (for example, reduced mycorrhizal activity), (Saravesi and others 2015; Parker and others 2017), a mechanistic understanding of underlying processes is still lacking and eventual consequences for soil C stocks are unknown.
In this study, we hypothesized that decomposition of soil OM and associated soil C losses via heterotrophic respiration would decelerate after moth outbreaks relative to the induced disturbance level (Figure 1). We related this to a decrease in tree root and ectomycorrhizal abundance and activity, an increase in soil N and an accompanied reduction in soil enzymatic activity (as outlined above). As a consequence of reduced soil C losses, we further hypothesized that soil C stocks of subarctic birch forest ecosystems would not decline significantly within the first decade after moth outbreaks. To test our hypotheses, we conducted a field experiment along five moth outbreak gradients in subarctic mountain birch stands in Norway, Finland and Sweden. The experiment covered a geographical range of approximately 500 km; soil CO2 efflux, heterotrophic respiration and enzymatic activity (as indices for decomposition), as well as root biomass, N availability and soil C stocks, were measured at 55 plots. Structural equation modelling was used to untangle complex relations among measured variables and to test how they are affected by disturbance from moth outbreaks.
Predicted effects of disturbance level by moth outbreaks on belowground properties and processes in subarctic mountain birch forests in Fennoscandia. A reduced carbon fixation by trees may be associated with a reduction in roots and ectomycorrhizal symbionts (EM). Soil nitrogen availability was expected to increase. Since enzymatic activity was supposed to decrease, decomposition of soil organic matter and an associated soil C loss were hypothesized to decrease after moth outbreaks (Color figure online).
Methods
Field Sites and Experimental Set-Up
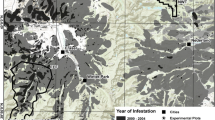
Soil CO2 efflux measurements and soil/root samples were taken in August 2013 at 5 mountain birch (Betula pubescens ssp. czerepanovii (N. I. Orlova) Hämet-Ähti) sites featuring different levels of moth outbreaks (‘disturbance levels’) and points of time post-disturbance. The sites were located in northern Norway, Finland and Sweden (Figure 2A). The north-western sites (Nor2, Fin1 and Fin2) were affected by outbreaks in 2006–08, the north-eastern site (Nor1) in 2007–09, and in the Swedish site the outbreak started in 2012 and was ongoing in 2013 during sampling (Figure 2B). The outbreaks are documented by a time series of the mean decrease in the Normalized Density Vegetation Index (NDVI), recorded around midsummer, within a 1-km buffer around all affected (intermediate, dead) plots, relative to a year without outbreaks (Figure 2B; Jepsen and others 2009a, b). The sampling areas in Norway, Finland and Sweden are dominated by well-developed podzols and are described in detail by Vindstad and others (2014), Kaukonen and others (2013), Parker and others (2017), and Olsson and others (2016), respectively.
Moth disturbance gradients and moth outbreak history. A A map of the study region in northern Fennoscandia with the 5 study sites marked, and B disturbance history per site as illustrated by a time series of the average NDVI anomaly within a 1-km buffer around all affected (intermediate, dead) plots. More negative values indicate a larger anomaly (for example, more severe outbreaks) (Color figure online).
At each site, 8–13 sampling plots were selected, representing two to three disturbance levels induced by moth outbreaks (Supplementary Figure 1): plots in stands undisturbed by moths (control, n = 23), plots with brief moth outbreaks and low tree mortality (intermediate, n = 12) and plots with extended moth outbreaks and very high tree mortality (dead, n = 20). At each plot, three subplots within 5 m from the centre were selected; criteria were a distance of more than 1.5 m to tree boles and more than 0.5 m to stone outcrops. Moth outbreak levels at plot/subplot level were homogeneous. The vegetation cover of grass and dwarf shrubs was estimated within a 0.5 m × 0.5 m square at each subplot. The ericaceous understory consisted of the dwarf shrubs Empetrum hermaphroditum, Vaccinium myrtillus, V. vitis-idaea, V. uliginosum; grass, especially Deschampsia flexuosa, dominated on severely disturbed plots. In total, 165 subplots were sampled, covering a wide range of abiotic and biotic conditions. Further information on sampling sites is given in Supplementary Table 2.
Soil CO2 Efflux
Soil CO2 efflux was measured in situ for 2 min or until ΔCO2 increase exceeded 50 ppm, using a closed chamber system with a respiration chamber of 10 cm in diameter (SRC-1, PP Systems, Amesbury, MA, USA) connected to an infrared gas analyser (IRGA; EGM 4, PP Systems). Measurements were conducted by placing the respiration chamber on the soil surface. Prior to measurements, aboveground vegetation was clipped to exclude aboveground organs from the measurement chamber and to guarantee chamber closure towards the ground. The effect of clipping on current autotrophic respiration was predicted negligible since grass root respiration has been shown to be largely maintained by carbohydrate reserves (Bahn and others 2006). Soil CO2 efflux measurements were repeated twice (interrupted by venting the chamber); if the soil CO2 efflux measurements differed more than 0.1 μmol CO2 m−2 s−1, a third measurement was conducted after venting. Soil temperature (± 0.1°C) was determined adjacent (4–5 cm) to the respiration chambers’ outer rim at 5 cm depth. Plot-specific temperature sensitivities obtained from heterotrophic respiration measurements ex situ (see below) were used to standardize field soil CO2 efflux to 10°C soil temperature (Curiel Yuste and others 2005); soil CO2 efflux in situ was expressed in µmol CO2 m−2s−1.
Soil Sampling and Sample Preparation
Per subplot, one soil core was extracted from underneath the spot of CO2 measurement—driving a soil corer (6.8 cm inner diameter) manually to a maximum soil depth of 20 cm (depending on stone content). The thickness of the humus layer was measured in the hole to correct for compression. The soil core was divided into humus layer and mineral soil and stored in plastic bags; mineral soil was divided into mineral soil of the first 10 cm not being humus (x–10 cm) and 10–20 cm soil depth. In the evening of each sampling day, the mineral soil samples were sieved (2 mm), and the stone content was recorded. Roots were collected and stored in water-filled plastic bags. The humus layer was stored in plastic bags until root collection and sieving (4 mm) took place in the laboratory. All soil and root samples were stored in a cooling room at 4°C until further analyses.
Root and Soil Analysis
Roots of soil depth 0–10 cm were rinsed and dissected into coarse (diameter > 2 mm; data not shown) and fine (d ≤ 2 mm) root segments; rarely discovered stolons of grass were discarded. Using a stereo microscope (10–40×), the fine root fraction was separated into woody and grass/herb roots (Rewald and others 2012); woody fine roots were further separated into living (biomass) and dead (necromass) root segments according to colour, root elasticity and the degree of cohesion of cortex, periderm and stele (Rewald and Leuschner 2009). All grass/herb roots were considered alive because of the relatively fast decay compared to woody roots. Subsequently, all root samples were dried (70°C, 48 h) and weighed (± 0.1 mg) to determine the root mass per m2.
After grinding (Pulverisette 5, Fritsch, Germany) and homogenization of the sieved humus layer, the total C and N concentrations of 300 mg of dried sub-samples were measured with a TruSpec CN analyser (Leco Corp., St Joseph, MI, USA) according to Austrian standard protocol (ÖNORM L 1080 1999). The organic content of the mineral soil was analysed by loss of ignition at 450°C (De Vos and others 2005); carbon content of mineral soil was calculated using measurements of 10 random samples by the TruSpec CN analyser as reference. Soil moisture content was calculated after drying at 70°C (until constant weight).
Soil organic C stocks (kg m−2) were determined for humus, 0–10 and 0–20 cm soil depth, respectively. When the soil corer did not reach 20 cm soil depth (due to high skeletal structure), the missing soil fraction of individual cores was predicted to hold the same C content as the sampled one.
Heterotrophic Respiration from Humus
The CO2 efflux from heterotrophic respiration was measured from composite humus samples using a closed chamber system. Composite samples were obtained by combining sieved humus samples of the three subplots per plot in equal proportions. Fresh humus of standardized moisture was filled into 250 cm3 plastic cores that were placed in sealed plastic containers (2 l). Inside an incubator, twenty containers were connected to an IRGA (SBA-4, PP Systems, USA); for measurement, a multiplexer switched between the containers every 10 min, while the 19 non-measured containers were vented (preventing CO2 build-up). CO2 efflux was measured consecutively at 5, 12.5, 20, 12.5 and 5°C; each temperature step lasted for 13 h (see Mayer and others 2017b for details). Heterotrophic respiration rates were related to incubation temperature, and temperature sensitivities were calculated; temperature sensitivities were expressed as Q10 values (that is, proportional change in heterotrophic respiration at a 10°C increase in temperature) as described in Mayer and others (2017a). Plot-specific temperature sensitivities were used to standardize field soil CO2 efflux at 10°C soil temperature (see above). Heterotrophic respiration rates of humus were expressed in µmol CO2 g C−1 s−1.
Humus Enzymatic Activities and N Concentrations
The three humus samples from each plot were merged (as above), and analysed for potential soil extracellular enzyme activity and N concentrations. Microbial extracellular enzyme activities were measured after incubation at room temperature using a microplate method (Allison and others 2008). A soil homogenate (3 g fresh soil, 60 ml Milli-Q water) was prepared, after which 200 µl of the sample was mixed with 100 µl of enzyme substrate in a 96-well plate: paranitrophenyl pNP-β-glucopyranoside for β-glucosidase (BG), pNP-β-N-acetylglucosaminide for β-N-acetylglucosaminidase (NAG, “Chitinase”), leucine p-nitroanilide for leucine aminopeptidase (LAP), pNP-phosphate for acid phosphatase (AP) and L-3,4-dihydroxyphenylalanine for phenol oxidase (PO). BG, NAG, LAP and AP catalyse reactions that hydrolyse the terminal linkages of oligomers released from polymers, and PO catalyses oxidative reactions in the decomposition of phenols; see Sinsabaugh and others (2008) for details. Urease (U) releases ammonium from urea (Carreiro and others 2000). Samples were analysed on a Multiskan FC microplate photometer (Thermo Fisher Scientific). Urease activity was verified by measuring the formation of NH4+ after 5 h (Carreiro and others 2000). Extinction coefficients were obtained based on standard curves for p-nitrophenol (BG, NAG, AP), p-nitroaniline (LAP) and NH4Cl (U); the coefficient for PO was determined as the oxidation of pyrogallol by mushroom tyrosinase. The background absorbance of the soil slurry and enzyme substrates was accounted for. Extracellular enzyme activities were calculated as µmol h−1 g−1 OM; organic matter was determined by loss on ignition (550°C, 3 h).
Inorganic N was analysed from two sub-samples of about 3 g humus which were extracted with 50 ml of 0.5 M K2SO4. Additionally, the concentrations of ammonium N (NH4; SFS 3032, Shimadzu UV-1700 spectrophotometer) and nitrate N (NO3; SFS-EN ISO 13395, CFA Seal Analytical AA3) were analysed.
Statistics and Structural Equation Modelling
Statistical analyses were conducted on plot means (n = 55) calculated from 3 subplots each. Linear mixed effect models (Pinheiro and Bates 2000) were used to test for effects on measured variables. Sites and moth outbreak levels (that is, disturbance levels; control, intermediate, dead) were, respectively, assigned as random and fixed effects in each model. Data were tested for normal distribution and variance homogeneity prior to analysis. If the assumption of homogeneity was violated, a disturbance-level specific variance structure was incorporated in the model (Zuur and others 2009). Tukey’s post hoc tests were used to analyse whether measured variables differed between disturbance levels.
Structural equation modelling (SEM) (Grace 2006; Grace and Bollen 2008; Beaujean 2014) was used to study relations among variables which were significantly affected by moth outbreaks. An a priori model was set up first (Grace 2006; Grace and Bollen 2008; Beaujean 2014). This a priori model included pathways which we hypothesized to describe the relations among disturbance level (by moth outbreaks), grass and woody fine root biomass, humus C/N ratio and NH4 concentration, potential enzyme activities of NAG and PO, heterotrophic respiration and soil CO2 efflux (Supplementary Figure 4). The variables C/N ratio and NH4 concentration were combined in a latent variable named ‘N availability’ (Beaujean 2014). The a priori model was tested against measurements, but many pathways and the overall model fit were not significant. Thus, in a backward model selection, the non-significant pathways were removed in order to obtain the best model. Model selection was based on Akaike’s information criterion (AIC), Chi-squared test results and Comparative Fit Index (CFI) (Grace and Bollen 2008; Beaujean 2014). The best model describing the relations among variables was identified by a low Chi-squared value, a high CFI and a model p value > 0.05 (indicating a good model fit in SEM).
Statistical analysis was performed in R (R Core Team 2014) using packages ‘nlme’ (Pinheiro and others 2014) and ‘lavaan’ (Rosseel 2012) for mixed effects modelling and structural equation modelling, respectively. Throughout the text, means and standard errors (SE) are given.
Results
Understory Vegetation, Root Biomass, Soil Temperature and Moisture
The three disturbance levels caused by absence/severity of moth outbreaks (control, intermediate and dead) featured significantly different understory compositions; dwarf shrubs prevailed at the control plots (65% surface cover), whereas the ground vegetation of dead plots was grass dominated (55% surface cover; Table 1). Compared to control plots, biomass of woody fine roots (d < 2 mm) was significantly lower at intermediate and dead plots; no differences were found between the latter (Table 1). The grass/herb root biomass showed the opposite trend, featuring significantly greater root biomasses at the dead plots than in the control plots (Table 1). Neither the bio-/necromass of woody coarse roots nor the necromass of woody fine roots differed between moth outbreak levels (data not shown). At the time of soil CO2 efflux measurements, soil moisture (humus layer) and soil temperature (at 5 cm depth) were 7.2% and 0.8°C higher at the dead plots as compared to the control plots (Table 1). Soil temperature of intermediate plots was found to be between those of control and dead plots.
Soil CO2 Efflux and Heterotrophic Respiration
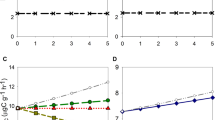
Soil CO2 efflux was significantly higher at control plots compared to intermediate and dead plots at all sites; no difference in soil CO2 efflux was found between intermediate and dead plots. (Figure 3A, Supplementary Figure 2). Heterotrophic respiration rates of the humus layer ex situ were found to be significantly higher at the control than at the intermediate plots; however, heterotrophic respiration rates at dead plots did not differ significantly from rates at control and intermediate plots, respectively (Figure 3B). Q10 did not vary among disturbance levels (data not shown).
Soil CO2 efflux and heterotrophic respiration of the humus layer. A Soil CO2 efflux measured in the field, and B heterotrophic respiration of the humus layer measured in the laboratory of control (not affected by moth outbreaks), intermediate (brief outbreaks and low tree mortality) and dead (extended outbreaks and very high tree mortality). Values have been standardized to fluxes at 10°C soil temperature. Significant differences between control, intermediate and dead stands are indicated by different letters (Tukey test, p < 0.05; n = 12–23, mean ± SE).
Soil C and N
Neither soil C content nor total C stocks were significantly affected by moth disturbance across soil horizons (Table 1), even when excluding the recently disturbed sites in Abisko (data not shown). The C/N ratio of the humus layer was significantly lower at the intermediate and dead plots as compared to the control plots; no difference in C/N ratios was found between intermediate and dead plots. This was reflected by the extractable NH4 concentrations of the humus layer, which were about three times higher in the dead plots as compared to the control plots (Table 1). Similarly, extractable NO3 concentration doubled in the humus layer of dead plots; however, this increase was not significant.
Potential Soil Enzymatic Activities
The potential enzyme activities of PO (that is, breakdown of non-easy degradable SOM compounds) and NAG (that is, breakdown of chitin from fungal cell walls) showed a clear decrease with level of disturbance and were significantly lower in intermediate and dead stands as compared to control (Figure 4); the difference in NAG between control and dead stands was, however, only marginal significant (p = 0.051). No differences in PO and NAG were detected between intermediate and dead plots. The potential activities of LAP, U, BG and AP were not significantly affected by moth outbreaks (Supplementary Figure 2a–d).
Potential enzymatic activities of PO and NAG as affected by moth outbreaks. Potential enzymatic activity of A peroxidase (PO) and B chitinase (NAG) at the 3 disturbance levels control (not affected by moth outbreaks), intermediate (brief outbreaks and low tree mortality) and dead (extended outbreaks and very high tree mortality). Significant differences between control, intermediate and dead stands are indicated by different letters (Tukey test, p < 0.05; n = 12–23, mean ± SE).
Structural Equation Model
The structural equation model (SEM; Figure 5) was based on the variables significantly affected by moth outbreaks (Table 1, Figures 3, 4). The final SEM (Figure 5) possessed a non-significant p-value (p = 0.25), a high CFI value (CFI = 0.98) and a low Chi-squared value (χ2 = 26.03), indicating a good fit to the data (Grace 2006; Beaujean 2014). The SEM explained 21–71% of the variation depending on the variable, soil CO2 efflux (62%), woody fine root biomass (33%), grass root biomass (21%), heterotrophic respiration (40%), N availability (37%), PO (66%) and NAG (71%). The disturbance level was positively related to grass/herb root biomass ① and negatively related to woody fine root biomass ②. (Numbers represent pathways in Figure 5, Supplementary Table 1.) A negative correlation was found between root types, that is, with increasing disturbance level the woody fine root biomass decreases and the grass/herb biomass increases ⑫. The N availability was negatively correlated with the woody fine root biomass ⑤. When the woody fine root biomass decreased (after disturbance), the increased N availability was based on a reduced C/N ratio and a greater NH4 concentration. The N availability was negatively related to potential NAG ⑥ and PO ⑦ activities. Thus, with increasing disturbance, the potential NAG (chitin hydrolysing) and PO (phenol oxidizing) activities decrease due to the increased N availability. A direct negative effect between disturbance and potential PO ③ and NAG ④ activities further enhanced the effect of disturbance on NAG and PO. Heterotrophic respiration was positively related to the potential PO activity ⑧, that is, when the potential PO activity decreased due to disturbance, the heterotrophic respiration decreased as well. Finally, the soil CO2 efflux measured in the field was positively related to woody fine root biomass ⑪, heterotrophic respiration ⑩ and potential NAG activity ⑨. Thus, the three major factors underlying the decreased soil CO2 efflux of disturbed plots were a reduced woody fine root biomass, a reduced heterotrophic respiration and a reduced potential NAG activity. Other tested relations included in the a priori model (Supplementary Figure 4) were not found to be significant or did not improve the overall accuracy of the final model (data not shown).
Structural equation model describing the effect of disturbance level (by moth outbreaks) on soil properties and processes and their relationship among each other. Solid boxes represent measured variables, and ellipses are latent variables (indicated by measured variables). Significant relationships and correlations between variables are represented by single- and double-headed arrows, respectively; solid (black) lines and dashed-dotted (red) lines are positive and negative (cor-)relations, respectively. Path coefficients are given in Supplementary Table 1. N nitrogen; PO phenol oxidase; NAG chitinase (Color figure online).
Discussion
Forest soils store huge amounts of carbon (C) and represent a globally important sink for atmospheric CO2 (Pan and others 2011; Bradshaw and Warkentin 2015). In the light of climate change, it is crucial to understand how different disturbance agents affect the forest C cycle. Here, we investigated soil properties and processes related to OM decomposition and soil C storage along moth disturbance gradients in subarctic mountain birch forests of Fennoscandia. Our indices/proxies for decomposition (for example, phenol oxidase, soil CO2 efflux) support our hypothesis that OM breakdown decreases with moth outbreak severity and accompanied disturbance levels (Figure 1). Consequently, reduced rates of decomposition were suggested to be a major cause for stable soil C stocks in moth-affected stands (Table 1).
We found a persistent decline in woody fine root biomass with increasing disturbance from moth outbreaks (Table 1), presumably related to a reduced C fixation after defoliation (Heliasz and others 2011; Dahl and others 2017; Olsson and others 2017). A decrease in woody fine roots has been reported previously for moth-attacked birch stands and other tree species after insect infestations (Borkhuu and others 2015; Cigan and others 2015; Saravesi and others 2015). A coinciding reduction in N uptake by root systems (Kosola and others 2001; Manninen and others 2011) and a slowed down N mobilization and uptake by ectomycorrhizas (Kaukonen and others 2013; Parker and others 2017) likely underlie the increase in N availability following moth outbreaks (Table 1, Figure 5, ⑤). At the same time, the N bound in the dying roots and mycorrhiza might get mineralized. In addition, the input from moth frass deposition likely contributes to higher levels of N immediately after defoliation (Kaukonen and others 2013). Although grass cover increased significantly with disturbance level (Figure 5, ①), we could not detect a direct link between grass root biomass and soil N availability (Figure 5). This suggests that N uptake by post-disturbance ground vegetation could not fully compensate for N accumulation following moth outbreaks.
Previous studies have shown that the microbial release of extracellular enzymes mediating soil OM decomposition is highly sensitive to N availability (Ramirez and others 2012; Bödeker and others 2014). Reduced activities in both PO (oxidizing phenols) and NAG (hydrolysing chitin) following moth outbreaks (Figure 4) could thus indicate a decrease in organic matter decomposition resulting from lower microbial N mining (Craine and others 2007; Baskaran and others 2017). This hypothesis of reduced decomposition is supported by the direct positive link between PO and heterotrophic respiration (Figure 5, ⑧), our measure for mineralization of organic C. Lower PO and NAG activities might also be related to a reduction in ectomycorrhizal fungi after birch defoliation (Saravesi and others 2015; Parker and others 2017). PO and NAG are enzymes commonly produced by fungi (Miller and others 1998; Sinsabaugh 2010), and particularly, NAG has been found to be strongly related to soil fungal biomass (Miller and others 1998). Many ectomycorrhizal species have low enzymatic capacity, especially of oxidizing enzymes, as compared to saprophytes (Pellitier and Zak 2018). However, in the mor-humus of boreal forests, the oxidative enzymatic potential seems to be strongly linked to the presence of ectomycorrhiza as reported earlier (Lindahl and Tunlid 2015; Sterkenburg and others 2018). In support, the most common (that is, 50% relative abundance) ectomycorrhizal species in Abisko, Cortinarius spp. (Parker and others 2017), have been shown to have a high PO activity, which decreases when N availability increases (Bödeker and others 2014)—and obviously also when mycorrhizas die off. Both a down-regulation of microbial N mining and a decrease in ectomycorrhizas due to tree death may therefore contribute to a decrease in decomposition after moth outbreaks. Moreover, decreasing decomposition rates might also be caused by stoichiometric constraints—soil microbes in subarctic regions have previously been shown to be co-limited by C and N (Demoling 2007; Sistla and others 2012). A reduced input of easy degradable substances from above- and belowground litter and an accompanied depletion in metabolically valuable OM could thus decelerate microbial activity. This is in line with Janssens and others (2010), who showed that adding N to easily decomposable organic material resulted in increased decomposition, whereas decomposition decreased if N was added to more recalcitrant OM such as humus.
Our hypothesis of decreased decomposition rates was also supported by the reduced in situ soil CO2 efflux following moth outbreaks, which dropped drastically even after intermediate moth disturbance (Figure 3A). The direct relation between heterotrophic respiration and soil CO2 efflux suggests that CO2 from decomposition is an important contributor to total respiration from soil (Figure 5, ⑩). However, a strong positive relation between woody fine roots and soil CO2 efflux also suggests a large decline in autotrophic respiration from birch and dwarf shrub roots after moth outbreaks. Moreover, the direct link between NAG activity and soil CO2 efflux (Figure 5, ⑨) might indicate a direct respiratory contribution from ectomycorrhizal fungi (Miller and others 1998; Heinemeyer and others 2007). Although no relation between grass/herb root biomass and soil CO2 efflux was evident from the structural equation model, it is, however, likely that the negative correlation between grass/herb and woody fine root biomasses (Figure 5, ⑫) accounted for a contribution by grasses.
We could not detect a change in soil C stocks along the investigated moth disturbance gradients (Table 1). This is in direct contradiction to earlier findings from global syntheses where a broad range of natural disturbances were shown to negatively affect soil C stocks in temperate and boreal forest ecosystems (Thom and Seidl 2015; Zhang and others 2015). We propose that reduced decomposition rates together with litter inputs from upcoming ground vegetation, remaining trees/shrubs and possibly also from dead tree compartments, kept effects on post-disturbance soil C stocks low for several years after the outbreak. Moreover, temperature might be a further reason for our contradicting results compared to other disturbed forest systems. Although in temperate forest ecosystems, a post-disturbance decline in soil C stocks was attributed to warmer soil conditions and increased decomposition rates (Christophel and others 2015; Mayer and others 2017b), we expect temperature changes after moth outbreaks to be of minor importance for soil C turnover. Although soil temperature in our study was 0.8°C higher in the dead stands than in the control (Table 1), more thorough measurements over the year have shown that subarctic birch forest soils are on average warmer than areas without trees (Karlsson and Weih 2001; Sjögersten and Wookey 2009). This suggests that even taking the effect of temperature into account, decomposition would decrease when the trees die off. Because changes in plant community are known to affect soil C stocks (Wardle and others 2012; Clemmensen and others 2015), a persistent shift from tree-dominated to grass-dominated ecosystems might have additional, unpredictable consequences on the soil C sequestration in subarctic regions. Tundra ecotones dominated by heath, for example, were shown to have larger soil C stocks than birch forests (Sjögersten and Wookey 2009; Parker and others 2015). In the long term, it might therefore be possible that soil C sequestration even increases following moth outbreak-induced vegetation shifts. Thus, further studies on long-term effects of moth outbreaks in Fennoscandia are urgently required.
Conclusions
This study suggests that decomposition of soil organic matter can slow down within the first decade following moth outbreaks in subarctic mountain birch forests. We propose that a higher soil N availability and an accompanied decrease in microbial N limitation as well as a general decrease in ectomycorrhizal fungi in disturbed birch stands were key drivers underlying the decline in soil extracellular enzymes involved in the breakdown of soil organic matter. Since soil C stocks remained unchanged (up to 8 years after tree dieback), our results suggest that the disturbance effect on plant C fixation and litter input was balanced by a decline in decomposition. A positive feedback on climate change by moth disturbance, via CO2 release of soil C, might therefore be smaller in subarctic birch forests than in disturbed forest ecosystems at lower latitudes (Bonan 2008; Kurz and others 2008).
References
Allison SD, Czimczik CI, Treseder KK. 2008. Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Global Change Biology 14:1156–68.
Averill C, Waring B. 2018. Nitrogen limitation of decomposition and decay: how can it occur? Global Change Biology 24:1417–27.
Bahn M, Knapp M, Garajova Z, Pfahringer N, Cernusca A. 2006. Root respiration in temperate mountain grasslands differing in land use. Global Change Biology 12:995–1006.
Baskaran P, Hyvönen R, Berglund SL, Clemmensen KE, Ågren GI, Lindahl BD, Manzoni S. 2017. Modelling the influence of ectomycorrhizal decomposition on plant nutrition and soil carbon sequestration in boreal forest ecosystems. New Phytologist 213:1452–65.
Beaujean AA. 2014. Latent variable modeling using R. New York: Routledge.
Bödeker I, Clemmensen KE, Boer W, Martin F, Olson Å, Lindahl BD. 2014. Ectomycorrhizal Cortinarius species participate in enzymatic oxidation of humus in northern forest ecosystems. New Phytologist 203:245–56.
Bonan GB. 2008. Forests and climate change: forcings, feedbacks, and the climate benefits of forests. Science 320:1444–9.
Borkhuu B, Peckham SD, Ewers BE, Norton U, Pendall E. 2015. Does soil respiration decline following bark beetle induced forest mortality? Evidence from a lodgepole pine forest. Agricultural and Forest Meteorology 214:201–7.
Bradshaw CJA, Warkentin IG. 2015. Global estimates of boreal forest carbon stocks and flux. Global and Planetary Change 128:24–30.
Brzostek ER, Dragoni D, Brown ZA, Phillips RP. 2015. Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytologist 206:1274–82.
Carreiro M, Sinsabaugh R, Repert D, Parkhurst D. 2000. Microbial enzyme shifts explain litter decay responses to simulated nitrogen deposition. Ecology 81:2359–65.
Christophel D, Höllerl S, Prietzel J, Steffens M. 2015. Long-term development of soil organic carbon and nitrogen stocks after shelterwood- and clear-cutting in a mountain forest in the Bavarian limestone Alps. European Journal of Forest Research 134:623–40.
Cigan PW, Karst J, Cahill JF, Sywenky AN, Pec GJ, Erbilgin N. 2015. Influence of bark beetle outbreaks on nutrient cycling in native pine stands in western Canada. Plant and Soil 390:29–47.
Clemmensen KE, Finlay RD, Dahlberg A, Stenlid J, Wardle DA, Lindahl BD. 2015. Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytologist 205:1525–36.
Craine JM, Morrow C, Fierer N. 2007. Microbial nitrogen limitation increases decomposition. Ecology 88:2105–13.
Curiel Yuste J, Nagy M, Janssens IA, Carrara A, Ceulemans R. 2005. Soil respiration in a mixed temperate forest and its contribution to total ecosystem respiration. Tree Physiology 25:609–19.
Dahl MB, Priemé A, Brejnrod A, Brusvang P, Lund M, Nymand J, Kramshøj M, Ro-Poulsen H, Haugwitz MS. 2017. Warming, shading and a moth outbreak reduce tundra carbon sink strength dramatically by changing plant cover and soil microbial activity. Scientific Reports 7:16035.
De Vos B, Vandecasteele B, Deckers J, Muys B. 2005. Capability of loss-on-ignition as a predictor of total organic carbon in non-calcareous forest soils. Communications in Soil Science and Plant Analysis 36:2899–921.
Demoling F. 2007. Nutrient limitation of bacterial growth in soil. Lund: Department of Ecology, Lund University. p p124.
Grace J, Bollen K. 2008. Representing general theoretical concepts in structural equation models: the role of composite variables. Environmental and Ecological Statistics 15:191–213.
Grace JB. 2006. Structural equation modeling and natural systems. Cambridge: Cambridge University Press.
Heinemeyer A, Hartley IP, Evans SP, Carreira De La Fuente JA, Ineson P. 2007. Forest soil CO2 flux: uncovering the contribution and environmental responses of ectomycorrhizas. Global Change Biology 13:1786–97.
Heliasz M, Johansson T, Lindroth A, Mölder M, Mastepanov M, Friborg T, Callaghan TV. 2011. Quantification of C uptake in subarctic birch forest after setback by an extreme insect outbreak. Geophysical Research Letters 38:L01704. https://doi.org/10.1029/2010GL044733.
Högberg P, Nordgren A, Buchmann N, Taylor AFS, Ekblad A, Hogberg MN, Nyberg G, Ottosson-Lofvenius M, Read DJ. 2001. Large-scale forest girdling shows that current photosynthesis drives soil respiration. Nature 411:789–92.
IPCC. 2014. Climate change 2014: synthesis report. In: Pachauri RK, Allen MR, Eds. Contribution of working groups I, II and III to the fifth assessment report of the intergovernmental panel on climate change. Geneva: Core Writing Team. p 151.
Janssens I, Dieleman W, Luyssaert S, Subke J-A, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G. 2010. Reduction of forest soil respiration in response to nitrogen deposition. Nature Geoscience 3:315–22.
Jepsen J, Hagen S, Høgda K, Ims R, Karlsen S, Tømmervik H, Yoccoz N. 2009a. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sensing of Environment 113:1939.
Jepsen JU, Biuw M, Ims RA, Kapari L, Schott T, Petter O, Vindstad L, Hagen SB. 2013. Ecosystem impacts of a range expanding forest defoliator at the forest-Tundra ecotone. Ecosystems 16:561–75.
Jepsen JU, Hagen SB, Ims RA, Yoccoz NG. 2008. Climate change and outbreaks of the geometrids Operophtera brumata and Epirrita autumnata in subarctic birch forest: evidence of a recent outbreak range expansion. Journal of Animal Ecology 77:257–64.
Jepsen JU, Hagen SB, Karlsen S-R, Ims RA. 2009b. Phase-dependent outbreak dynamics of geometrid moth linked to host plant phenology. Proceedings of the Royal Society of London B: Biological Sciences 276:4119–28.
Kaiser C, Fuchslueger L, Koranda M, Gorfer M, Stange CF, Kitzler B, Rasche F, Strauss J, Sessitsch A, Zechmeister-Boltenstern S, Richter A. 2011. Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation. Ecology 92:1036–51.
Kaiser C, Koranda M, Kitzler B, Fuchslueger L, Schnecker J, Schweiger P, Rasche F, Zechmeister-Boltenstern S, Sessitsch A, Richter A. 2010. Belowground carbon allocation by trees drives seasonal patterns of extracellular enzyme activities by altering microbial community composition in a beech forest soil. New Phytologist 187:843–58.
Karlsson P, Weih M. 2001. Soil temperatures near the distribution limit of the mountain birch (Betula pubescens ssp. czerepanovii): implications for seedling nitrogen economy and survival. Arctic, Antarctic, and Alpine Research 33:88–92.
Kaukonen M, Ruotsalainen AL, Wäli PR, Männistö MK, Setälä H, Saravesi K, Huusko K, Markkola A. 2013. Moth herbivory enhances resource turnover in subarctic mountain birch forests? Ecology 94:267–72.
Kosola KR, Dickmann DI, Paul EA, Parry D. 2001. Repeated insect defoliation effects on growth, nitrogen acquisition, carbohydrates, and root demography of poplars. Oecologia 129:65–74.
Kurz WA, Dymond CC, Stinson G, Rampley GJ, Neilson ET, Carroll AL, Ebata T, Safranyik L. 2008. Mountain pine beetle and forest carbon feedback to climate change. Nature 452:987–90.
Kuuluvainen T, Hofgaard A, Aakala T, Gunnar Jonsson B. 2017. North Fennoscandian mountain forests: history, composition, disturbance dynamics and the unpredictable future. Forest Ecology and Management 385:140–9.
Lindahl BD, Tunlid A. 2015. Ectomycorrhizal fungi—potential organic matter decomposers, yet not saprotrophs. New Phytologist 205:1443–7.
Manninen O, Stark S, Kytöviita MM, Tolvanen A. 2011. Individual and combined effects of disturbance and N addition on understorey vegetation in a subarctic mountain birch forest. Journal of Vegetation Science 22:262–72.
Mayer M, Matthews B, Rosinger C, Sandén H, Godbold DL, Katzensteiner K. 2017a. Tree regeneration retards decomposition in a temperate mountain soil after forest gap disturbance. Soil Biology and Biochemistry 115:490–8.
Mayer M, Sandén H, Rewald B, Godbold DL, Katzensteiner K. 2017b. Increase in heterotrophic soil respiration by temperature drives decline in soil organic carbon stocks after forest windthrow in a mountainous ecosystem. Functional Ecology 31:1163–72.
Miller M, Palojärvi A, Rangger A, Reeslev M, Kjøller A. 1998. The use of fluorogenic substrates to measure fungal presence and activity in soil. Applied and Environmental Microbiology 64:613–17.
Neuvonen S, Niemelä P, Virtanen T. 1999. Climatic change and insect outbreaks in boreal forests: the role of winter temperatures. Ecological Bulletins: 47:63–67.
Normander B, Levin G, Auvinen A-P, Bratli H, Stabbetorp O, Hedblom M, Glimskär A, Gudmundsson GA. 2009. State of biodiversity in the Nordic countries—an assessment of progress towards achieving the target of halting biodiversity loss by 2010. Copenhagen Nordic Council of Ministers.
Olsson P-O, Heliasz M, Jin H, Eklundh L. 2017. Mapping the reduction in gross primary productivity in subarctic birch forests due to insect outbreaks. Biogeosciences 14:1703.
Olsson P-O, Lindström J, Eklundh L. 2016. Near real-time monitoring of insect induced defoliation in subalpine birch forests with MODIS derived NDVI. Remote Sensing of Environment 181:42–53.
ÖNORM L 1080. 1999. Chemische Bodenuntersuchungen—Bestimmung des organischen Kohlenstoffs durch trockene Verbrennung. Austrian Standards Institute.
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG, Ciais P, Jackson RB, Pacala SW, McGuire AD, Piao S, Rautiainen A, Sitch S, Hayes D. 2011. A large and persistent carbon sink in the world’s forests. Science 333:988–93.
Parker TC, Sadowsky J, Dunleavy H, Subke J-A, Frey SD, Wookey PA. 2017. Slowed biogeochemical cycling in sub-arctic birch forest linked to reduced mycorrhizal growth and community change after a defoliation event. Ecosystems 20:316–30.
Parker TC, Subke JA, Wookey PA. 2015. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Global Change Biology 21:2070–81.
Pellitier PT, Zak DR. 2018. Ectomycorrhizal fungi and the enzymatic liberation of nitrogen from soil organic matter: why evolutionary history matters. New Phytologist 217:68–73.
Pena R, Offermann C, Simon J, Naumann PS, Geßler A, Holst J, Dannenmann M, Mayer H, Kögel-Knabner I, Rennenberg H, Polle A. 2010. Girdling affects ectomycorrhizal fungal (EMF) diversity and reveals functional differences in EMF community composition in a beech forest. Applied and Environmental Microbiology 76:1831–41.
Pinheiro JC, Bates DM. 2000. Mixed-effects models in S and S-plus. New York: Springer. p 530.
Pinheiro JC, Bates DM, DebRoy S, Sarkar D, R Core team. 2014. nlme: Linear and nonlinear mixed effects models. R package version 3.1–117.
R Core Team. 2014. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
Ramirez KS, Craine JM, Fierer N. 2012. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Global Change Biology 18:1918–27.
Ramsfield T, Bentz B, Faccoli M, Jactel H, Brockerhoff E. 2016. Forest health in a changing world: effects of globalization and climate change on forest insect and pathogen impacts. Forestry 89:245–52.
Rewald B, Leuschner C. 2009. Belowground competition in a broad-leaved temperate mixed forest: pattern analysis and experiments in a four-species stand. European Journal of Forest Research 128:387–98.
Rewald B, Meinen C, Trockenbrodt M, Ephrath JE, Rachmilevitch S. 2012. Root taxa identification in plant mixtures—current techniques and future challenges. Plant and Soil 359:165–82.
Rosseel Y. 2012. lavaan: an R package for structural equation modeling. Journal of Statistical Software 48:1–36.
Saravesi K, Aikio S, Wäli PR, Ruotsalainen AL, Kaukonen M, Huusko K, Suokas M, Brown SP, Jumpponen A, Tuomi J. 2015. Moth outbreaks alter root-associated fungal communities in subarctic mountain birch forests. Microbial Ecology 69:788–97.
Scott-Denton LE, Rosenstiel TN, Monson RK. 2006. Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Global Change Biology 12:205–16.
Sinsabaugh RL. 2010. Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biology and Biochemistry 42:391–404.
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME. 2008. Stoichiometry of soil enzyme activity at global scale. Ecology Letters 11:1252–64.
Sistla SA, Asao S, Schimel JP. 2012. Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biology & Biochemistry 55:78–84.
Sjögersten S, Wookey PA. 2009. The impact of climate change on ecosystem carbon dynamics at the Scandinavian mountain birch forest-Tundra heath ecotone. AMBIO: A Journal of the Human Environment 38:2–10.
Sterkenburg E, Clemmensen KE, Ekblad A, Finlay RD, Lindahl BD. 2018. Contrasting effects of ectomycorrhizal fungi on early and late stage decomposition in a boreal forest. The ISME Journal 12:2187–97.
Tenow O, Bylund H. 2000. Recovery of a Betula pubescens forest in northern Sweden after severe defoliation by Epirrita autumnata. Journal of Vegetation Science 11:855–62.
Thom D, Rammer W, Seidl R. 2017. The impact of future forest dynamics on climate: interactive effects of changing vegetation and disturbance regimes. Ecological Monographs 87:665–84.
Thom D, Seidl R. 2015. Natural disturbance impacts on ecosystem services and biodiversity in temperate and boreal forests. Biological Reviews 91:760–81.
Vindstad OPL, Schultze S, Jepsen JU, Biuw M, Kapari L, Sverdrup-Thygeson A, Ims RA. 2014. Numerical responses of saproxylic beetles to rapid increases in dead wood availability following geometrid moth outbreaks in sub-arctic mountain birch forest. PloS One 9:e99624.
Virtanen T, Neuvonen S, Nikula A. 1998. Modelling topoclimatic patterns of egg mortality of Epirrita autumnata (Lepidoptera: geometridae) with a geographical information system: predictions for current climate and warmer climate scenarios. Journal of Applied Ecology 35:311–22.
Wardle DA, Jonsson M, Bansal S, Bardgett RD, Gundale MJ, Metcalfe DB. 2012. Linking vegetation change, carbon sequestration and biodiversity: insights from island ecosystems in a long-term natural experiment. Journal of Ecology 100:16–30.
Weintraub M, Scott-Denton L, Schmidt S, Monson R. 2007. The effects of tree rhizodeposition on soil exoenzyme activity, dissolved organic carbon, and nutrient availability in a subalpine forest ecosystem. Oecologia 154:327–38.
Zhang B, Zhou X, Zhou L, Ju R. 2015. A global synthesis of below-ground carbon responses to biotic disturbance: a meta-analysis. Global Ecology and Biogeography 24:126–38.
Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. New York: Springer.
Acknowledgements
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU). The authors are grateful for the hospitality and help at Abisko and Kevo research stations and NIBIO Svanhovd. The help of Felix Mayer during the field campaign and Ortal Rewald in the laboratory is highly appreciated. We want to thank Clementine Brakspear for providing the paintings of Figure 1.
Funding
This study was funded by the European Unions’ FP7 (Grant No. 262693 [INTERACT]), the Austrian Federal Ministry of Education, Science and Research (Project “Gemeinsam für nachhaltige Entwicklung – The Future we Want”) and Douglas L. Godbold, Institute of Forest Ecology, BOKU Vienna. JUJ was supported by the Research Council of Norway (Grant No. 244454) and PRW by the Academy of Finland (Grant No. 138309).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Author Contributions
HS initiated the project, performed the data analysis and wrote the manuscript together with MM, who also contributed the SEM model, and BR; TS, LON, SS, JUJ and PRW contributed to the writing; BR, TS, LON, HS and MM did the field-/laboratory work; SS analysed enzyme activities and nitrogen concentrations; and JUJ and PRW guided to the sites in Norway and Finland. HS and MM contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sandén, H., Mayer, M., Stark, S. et al. Moth Outbreaks Reduce Decomposition in Subarctic Forest Soils. Ecosystems 23, 151–163 (2020). https://doi.org/10.1007/s10021-019-00394-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00394-6