Abstract
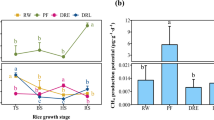
Natural peatlands are known as an important source of methane (CH4). However, most peatlands in Central Europe were drained for forestry purposes in the past and some of them have been recently restored, both having a strong impact on CH4 emissions. The main aim of our study was to determine the effect of long-term drainage (a few decades) and hydrological restoration (3 years) on CH4 emissions, potential CH4 production and on the methanogenic Archaea community in different types of peatlands under various hydrological regimes. For this purpose, CH4 emissions together with biotic and abiotic variables were measured over three growing seasons, in vitro potential CH4 production was determined, and qPCR and DGGE fingerprinting were used for methanogenic community description in three ombrotrophic bogs (pristine, drained, restored) and two fens (pristine, drained) located in the Šumava National Park within the Bohemian Forest, Czech Republic. The highest CH4 emissions, CH4 potential production, and the highest diversity of the methanogenic community were observed in the pristine fen and bog. In the frame of interannual variability, a drought period seemed to play an important role in seasonal CH4 fluxes. Plant species composition together with water table seemed to be the most important factors controlling CH4 emission. Drainage led to a significant decrease in CH4 emissions, potential CH4 production, and the abundance and diversity of methanogens as compared to pristine sites. These post-drainage changes were more obvious in the fen site than on bog sites. However, none of the measured parameters showed significant changes during the 3 years after rewetting. We assume that the restored hydrology was not the main factor controlling CH4 emissions and other factors such as vegetation composition and input of available substrate for methanogenic community were more important. Therefore it appears that the period necessary for regeneration of CH4 emissions is the result of restoring all of these elementary factors together.







Similar content being viewed by others
References
Aurela M, Ruitta T, Laurila T, Tuovinen J-P, Vesala T, Tuittila E-S, Rinne J, Haapanala S, Laine J. 2007. CO2 exchange of a sedge fen in southern Finland—the impact of a drought period. Tellus 59B:826–37.
Basiliko N, Yavitt JB, Dees PM, Merkel SM. 2003. Methane biogeochemistry and methanogens communities in two northern peatland ecosystems, New York State. Geomicrobiol J 20:563–77.
Basiliko N, Knowles R, Moore TR. 2004. Roles of moss species and habitat in methane consumption potential in a northern peatland. Wetlands 24:178–85.
Bergman I, Svensson BH, Nilsson M. 1998. Regulation of methane production in a Swedish acid mire by pH, temperature and substrate. Soil Biol Biochem 30:729–41.
Bergman I, Klarqvist M, Nilsson M. 2000. Seasonal variation in rates of methane production from peat of various botanical origins: effects of temperature and substrate quality. FEMS Microbiol Ecol 33:181–9.
Best EPH, Jacobs FHH. 1997. The influence of raised water table levels on carbon dioxide and methane production in ditch-dissected peat grasslands in the Netherlands. Ecol Eng 8:129–44.
Bufková I. 2009. Restoration as pre- non-intervention phase of mire management (Sumava National Park, Czech Republic). In: Huslein M, Kiener H, Křenová Z, Šolar M, Eds. The appropriateness of non-intervention management for protected areas and NATURA 2000 locations, Conference Report, 25–28th January 2009, Srní, Šumava National Park, pp 55–7.
Dias ATC, Hoorens B, Van Logtestijn RSP, Vermaat JE, Aerts R. 2010. Plant species composition can be used as a proxy to predict methane emissions in peatland ecosystems after land-use changes. Ecosystems 13:526–38.
Dowrick DJ, Freeman C, Lock MA, Reynolds B. 2006. Sulphate reduction and the suppression of peatland methane emissions following summer drought. Geoderma 132:384–90.
Forbrich I, Kutzbach L, Hormann A, Wilmking M. 2010. A comparison of linear and exponential regression for estimating diffusive CH4 fluxes by closed-chamber method. Soil Biol Biochem 42:507–15.
Freeman C, Hudson J, Lock MA, Reynolds B, Swanson C. 1994. A possible role of sulphate in the suppression of wetland methane fluxes following drought. Soil Biol Biochem 26:1439–42.
Galand PE, Saarnio S, Fritze H, Yrjälä K. 2002. Depth related diversity of methanogen Archaea in Finnish oligotrophic fen. FEMS Microbiol Ecol 42:441–9.
Galand PE, Fritze H, Conrad R, Yrjala K. 2005. Pathways for methanogenesis and diversity of methanogenic Archaea in three boreal peatland ecosystems. Appl Environ Microbiol 71:2195–8.
Hahn-Schöfl M, Zak D, Minke M, Gelbrecht J, Augustin J, Freibauer A. 2011. Organic sediment formed during inundation of degraded fen grassland emits large fluxes of CH4 and CO2. Biogeosciences 8:1539–50.
Hales BA, Edwards C, Ritchie DA, Hall G, Pickup RW, Saunders JR. 1996. Isolation and identification of methanogen-specific DNA from blanket bog peat by PCR amplification and sequence analysis. Appl Environ Microb 62:668–75.
Harriss RC, Gorham E, Sebacher DI, Bartlett KB, Flebbe PA. 1985. Methane flux from northern peatlands. Nature 315:652–4.
Hoj L, Rusten M, Haugen LE, Olsen RA, Torsvik VL. 2006. Effects of water regime on archaeal community composition in Arctic soils. Environ Microbiol 8:984–96.
Jerman V, Metje M, Mandic-Mulec I, Frenzel P. 2009. Wetland restoration and methanogenesis: the activity of microbial populations and competition for substrates at different temperatures. Biogeosciences 6:1127–38.
Joabsson A, Christensen TR, Wallén B. 1999. Vascular plant controls on methane emissions from northern peatforming wetlands. Trends Ecol Evol 14:385–8.
Kim SY, Lee SH, Freeman C, Fenner N, Kang H. 2008. Comparative analysis of soil microbial communities and their responses to the short-term drought in bog, fen, and riparian wetlands. Soil Biol Biochem 40:2874–80.
Komulainen V-M, Nykänen H, Martikainen PJ, Laine J. 1998. Short-term effect of restoration on vegetation change and methane emissions from peatlands drained for forestry in southern Finland. Can J For Res 28:402–11.
Komulainen V-M, Tuittila ES, Vasander H, Laine J. 1999. Restoration of drained peatlands in southern Finland: initial effects on vegetation chase and CO2 balance. J Appl Ecol 36:634–48.
Laiho R, Vasander H, Penttila T, Laine J. 2003. Dynamics of plant-mediated organic matter and nutrient cycling following long-term water-level drawdown in boreal peatlands. Global Biogeochem Cycles 17:1053. doi:10.1029/2002GB002015.
Mahmood MdS, Strack M. 2011. Methane dynamics of recolonized cutover minerotrophic peatland: implication for restoration. Ecol Eng 37:1859–68.
Minkkinen K, Laine J. 2006. Vegetation heterogeneity and ditches create spatial variability in methane fluxes from peatlands drained for forestry. Plant Soil 285:289–304.
Minkkinen K, Korhonen R, Savolainen I, Laine J. 2002. Carbon balance and radiative forcing of Finnish peatlands 1900–2100—the impact of forestry drainage. Glob Change Biol 8:758–99.
Moore TR, Young A, Bubier JL, Humphreys ER, Lafleur PM, Roulet NT. 2011. A multi-year record of methane flux at the Mer Bleue Bog, Southern Canada. Ecosystems 14:646–57.
Nykänen H, Alm J, Silvola J, Tolonen K, Martikainen PJ. 1998. Methane fluxes on boreal peatlands of different fertility and the effect of long-term experimental lowering of the water table on flux rates. Global Biogeochem Cycles 12:53–69.
Roulet NT, Moore TR. 1995. The effect of forestry drainage practices on the emission of methane from northern peatlands. Can J For Res 25:491–9.
Saarnio S, Alm J, Silvola J, Lohila A, Nykänen H, Mrtikainen PJ. 1997. Seasonal variation in CH4 emissions and production and oxidation potentials on an oligotrophic pine fen. Oecologia 110:414–22.
Saarnio S, Morer M, Shurpali NJ, Tuittila E-S, Mäkilä M, Alm J. 2007. Annual CO2 and CH4 fluxes of pristine boreal mires as a background for the lifecycle analyses of peat energy. Boreal Environ Res 12:101–13.
Schimel JP. 1995. Plant transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28:183–200.
Ström L, Ekberg A, Mastepanov M, Christensen TR. 2003. The effect of vascular plants on carbon turnover and methane emissions from a tundra wetland. Glob Change Biol 9:1185–92.
Ström L, Mastepanov M, Christensen TR. 2005. Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry 75:65–82.
Sundh I, Mikkelä C, Nilsson M, Svensson BH. 1995. Potential aerobic methane oxidation in a Sphagnum-dominated peatland—controlling factors and relation to methane emission. Soil Biol Biochem 27:827–37.
ter Braak CJF, Šmilauer P. 1998. CANOCO Reference Manual and User’s Guide to Canoco for Windows Microcomputer Power, Ithaca. 351 pp.
Tuittila E-S, Komulainen V-M, Vasander H, Nykanen H, Martikainen PJ, Laine J. 2000. Methane dynamics of a restored cut-away peatlands. Glob Change Biol 6:569–81.
Urbanová Z, Picek T, Bárta J. 2011. Effect of peat re-wetting on carbon and nutrient fluxes, greenhouse gas production and diversity of methanogenic archaeal community. Ecol Eng 37:1017–26.
Urbanová Z, Picek T, Hájek T, Bufková I, Tuittila E-S. 2012. Vegetation and carbon gas dynamics under a changed hydrological regime in central European peatlands. Plant Ecol Divers 5:89–103.
Waddington JM, Day SM. 2007. Methane emissions from a peatland following restoration. J Geophys Res Biogeosci 112:G03018.
Watanabe T, Asakawa S, Nakamura A, Nagaoka K, Kimura M. 2004. DGGE method for analyzing 16S rDNA of methanogenic archaeal community in paddy field soil. FEMS Microbiol Lett 232:153–63.
Wheeler BD, Shaw SC. 1995. Restoration of damaged peatlands with particular reference to lowland raised bogs affected by peat extraction. London: Department of the Environment, University of Sheffield.
Wilson D, Alm J, Riutta T, Laine J, Byrne KA, Farrell EP, Tuittila E-S. 2007. A high resolution green area index for modelling the seasonal dynamics of CO2 exchange, in peatland vascular plant communities. Plant Ecol 190:37–51.
Yavitt JB, Land GE. 1990. Methane production in contrasting wetland sites: response to organic-chemical components of peat and to sulfate reduction. Geomicrobiology 8:27–46.
Yavitt JB, Williams CJ, Wieder RK. 1997. Production of methane and carbon dioxide in peatland ecosystems across North America: effects of temperature, aeration, and organic chemistry of the peat. Geomicrobiol J 14:299–316.
Yrjälä K, Tuomivirta T, Juottonen H, Putkinen A, Lappi K, Tuittila E-S, Penttilä T, Minkkinen K, Laine J, Peltoniemi K, Fritze H. 2011. CH4 production and oxidation processes in a boreal fen ecosystem after long-term water table drawdown. Glob Change Biol 17:1311–20.
Acknowledgments
The study was supported by the Grant Agency of the Czech Republic, Project No. 526/09/1545, and the Czech Ministry of Environment, Project No. GAJU 143/2010/P. We thank students Jana Baxová, Eliška Jánská, Jiří Mastný and Annukka Närhi for their help in both the field and laboratory work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
Zuzana Urbanova conceived the study, performed research, analyzed data, wrote the paper. Jiri Barta responsible for molecular analysis qPCR and DGGE, analyzed data of the molecular analysis. Tomas Picek supervised the study, contributed to the paper writing.
Rights and permissions
About this article
Cite this article
Urbanová, Z., Bárta, J. & Picek, T. Methane Emissions and Methanogenic Archaea on Pristine, Drained and Restored Mountain Peatlands, Central Europe. Ecosystems 16, 664–677 (2013). https://doi.org/10.1007/s10021-013-9637-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-013-9637-4