Abstract
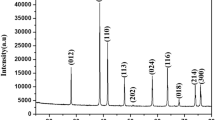
By use of a Hall measurement gaussmeter, the magnetic properties of chemically-reacting iron-sulfur powder mixtures and of iron powder plus hydrochloric acid have been extensively studied. In the case of iron sulfide (non-stoichiometric Fe1-xS) the measured value of the magnetic (B) field first increased continuously and significantly as the chemical reaction proceeded, then decreased sharply as the reaction neared completion. This behavior was stronger in the presence of an externally applied static magnetic field but was also clearly observed in the absence of an external field. The recovered product of the reaction was bronze-colored pyrrhotite which was in the form of a thin magnetic coating on Fe particles with a narrow conical morphology aligned with the external B field. The magnetic behavior during the Fe+HCl reaction was similar but far less pronounced. The general magnetic field behavior in both systems parallels the character of an activated state energy behavior.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 21 July 1998 / Accepted: 28 August 1998
Rights and permissions
About this article
Cite this article
Vezzoli, G., Forbes, J., Hurst, J. et al. Variation in magnetic properties during chemical reactions producing Fe1-xS and FeCl2 . Mat Res Innovat 2, 170–175 (1998). https://doi.org/10.1007/s100190050080
Issue Date:
DOI: https://doi.org/10.1007/s100190050080