Abstract
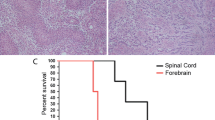
We previously reported that retrovirally transduced platelet-derived growth factor-B (PDGFB) in glial progenitors of the rat cerebral white matter, subventricular zone, or brain stem induced malignant brain tumors closely resembling human glioblastoma (GBM). While human GBMs may progress over the period of several months to a few years, prospective, long-term in-vivo observation of histological changes of the tumor tissues is not feasible in these models, because the animals undergo rapid tumor progression and mortality within approximately 1 month. We thus performed successive, long-term in-vivo transplantation of the PDGFB-induced tumor cells into the rat cerebrum. Primary retroviral transduction of PDGFB in the glial progenitors of the rat basal ganglia induced malignant glioma resembling human GBM or anaplastic oligodendroglioma (AOL) consisting of relatively monomorphous tumor cells expressing markers for the oligodendrocyte lineage. In the course of long-term successive transplantation, tumor cells presented pleomorphism as well as focal GFAP expression. This suggests that secondary chromosomal aberration and dysregulation of gene expression following accelerated cell cycle by PDGFB stimulation would induce morphological and immunophenotypic changes in tumor cells. Furthermore, while the primary tumors contained only a minor fraction of proviral GFP-expressing or hemagglutinin-expressing cells, most tumor cells came to express these proviral genes in the course of serial transplantation suggesting a persistent role of PDGFB-expressing cells in maintenance and growth of the tumors. This model would be useful for investigation of the long-term effects of PDGFB stimulation in glioma tissues on anaplastic evolution.




Similar content being viewed by others
References
Furnari FB, Fenton T, Bachoo RM et al (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710
Louis DN, Holland EC, Cairncross JG (2001) Glioma classification: a molecular reappraisal. Am J Pathol 159:779–786
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Di Rocco F, Carroll RS, Zhang J et al (1998) Platelet-derived growth factor and its receptor expression in human oligodendrogliomas. Neurosurgery 42:341–346
Nister M, Libermann TA, Betsholtz C et al (1988) Expression of messenger RNAs for platelet-derived growth factor and transforming growth factor-alpha and their receptors in human malignant glioma cell lines. Cancer Res 48:3910–3918
Hermanson M, Funa K, Hartman M et al (1992) Platelet-derived growth factor and its receptors in human glioma tissue: expression of messenger RNA and protein suggests the presence of autocrine and paracrine loops. Cancer Res 52:3213–3219
Armstrong RC, Harvath L, Dubois-Dalcq ME (1990) Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res 27:400–407
Frost EE, Zhou Z, Krasnesky K et al (2008) Initiation of oligodendrocyte progenitor cell migration by a PDGF-A activated extracellular regulated kinase (ERK) signaling pathway. Neurochem Res 34:169–181
Noble M, Murray K, Stroobant P et al (1988) Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of the oligodendrocyte/type-2 astrocyte progenitor cell. Nature 333:560–562
Bogler O, Wren D, Barnett SC et al (1990) Cooperation between two growth factors promotes extended self-renewal and inhibits differentiation of oligodendrocyte-type-2 astrocyte (O-2A) progenitor cells. Proc Natl Acad Sci USA 87:6368–6372
McKinnon RD, Matsui T, Dubois-Dalcq M et al (1990) FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron 5:603–614
Dai C, Celestino JC, Okada Y et al (2001) PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev 15:1913–1925
Hesselager G, Uhrbom L, Westermark B et al (2003) Complementary effects of platelet-derived growth factor autocrine stimulation and p53 or Ink4a-Arf deletion in a mouse glioma model. Cancer Res 63:4305–4309
Uhrbom L, Hesselager G, Nister M et al (1998) Induction of brain tumors in mice using a recombinant platelet-derived growth factor B-chain retrovirus. Cancer Res 58:5275–5279
Appolloni I, Calzolari F, Tutucci E et al (2009) PDGF-B induces a homogeneous class of oligodendrogliomas from embryonic neural progenitors. Int J Cancer 124(10):2251–2259
Calzolari F, Appolloni I, Tutucci E et al (2008) Tumor progression and oncogene addiction in a PDGF-B-induced model of gliomagenesis. Neoplasia 10:1373–1382
Assanah M, Lochhead R, Ogden A et al (2006) Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J Neurosci 26:6781–6790
Jackson EL, Garcia-Verdugo JM, Gil-Perotin S et al (2006) PDGFR alpha-positive B cells are neural stem cells in the adult SVZ that form glioma-like growths in response to increased PDGF signaling. Neuron 51:187–199
Assanah MC, Bruce JN, Suzuki SO et al (2009) PDGF stimulates the massive expansion of glial progenitors in the neonatal forebrain. Glia 57(16):1835–1847
Masui K, Suzuki SO, Torisu R et al (2010) Glial progenitors in the brainstem give rise to malignant gliomas by platelet-derived growth factor stimulation. Glia 58(9):1050–1065
Gensert JM, Goldman JE (2001) Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J Neurobiol 48:75–86
Mason JL, Goldman JE (2002) A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci 20:30–42
Fleming TP, Saxena A, Clark WC et al (1992) Amplification and/or overexpression of platelet derived growth factor receptors and epidermal growth factor receptor in human glial tumors. Cancer Res 52:4550–4553
Shih AH, Dai C, Hu X et al (2004) Dose-dependent effects of platelet derived growth factor-B on glial tumorigenesis. Cancer Res 64:4783–4789
Majumdar K, Radotra BD, Vasishta RK et al (2009) Platelet-derived growth factor expression correlates with tumor grade and proliferative activity in human oligodendrogliomas. Surg Neurol 72:54–60
Verhaak RGW, Hoadley KA, Purdom E et al (2010) Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 17:98–110
Uhrbom L, Nerio E, Holland EC (2004) Dissecting tumor maintenance requirements using bioluminescence imaging of cell proliferation in a mouse glioma model. Nat Med 10:1257–1260
Acknowledgments
The authors would like to thank Dr Kensuke Sasaki for instruction in statistical analysis, Dr Yoshihiro Seki for helpful comments and supports, and Ms Sachiko Nagae and Kimiko Sato for their excellent technical instruction and assistance (all from Department of Neuropathology, Kyushu University). They also thank Dr Hideki Horikawa (Department of Neuropsychiatry, Kyushu University) for instruction of ELISA, and Dr Sumako Nishimura and Dr Takeshi Yasunaga (Hitachi Medical Corporation) for their excellent technical assistance with open-MRI.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torisu, R., Suzuki, S.O., Masui, K. et al. Persistent roles of signal transduction of platelet-derived growth factor B in genesis, growth, and anaplastic transformation of gliomas in an in-vivo serial transplantation model. Brain Tumor Pathol 28, 33–42 (2011). https://doi.org/10.1007/s10014-010-0006-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-010-0006-0