Abstract
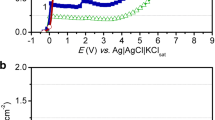
Titanium dissolution and passivation were studied in NaOH aqueous solution using open-circuit potential, potentiodynamic and potentiostatic techniques. Potentiodynamic data showed that the active-passive transition involves active metal dissolution followed by formation of a poorly conducting passive oxide film that passivates the electrode. The critical current density varied with pH as d log<I> j</I><SUB>m</SUB>/d pH=-0.098 in the pH range 11.00–14.00, while the passivation potential is changed according to the following two features: at pH 10.55–13.00, d<I>E</I><SUB>m</SUB>/d pH=-0.06 V; and at pH 13.50–14.00, d<I>E</I><SUB>m</SUB>/d pH=-0.40 V. The apparent activation energy, E*, was calculated from the slope of the Arrhenius plot and was found to be 12.6 kJ mol–1. Current-time transients showed that the growth of titanium oxide passive film is a diffusion-controlled process. XPS measurements indicated that the passive oxide film consists mainly of TiO2 and a mixture of suboxides of Ti2O3 and TiO.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Ibrahim, M.A., Pongkao, D. & Yoshimura, M. The electrochemical behavior and characterization of the anodic oxide film formed on titanium in NaOH solutions. J Solid State Electrochem 6, 341–350 (2002). https://doi.org/10.1007/s100080100229
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s100080100229