Abstract
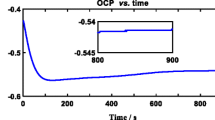
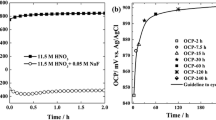
The stability of electrochemically formed NiF2 film in 1.0 M perchloric acid containing monovalent fluorides namely, NH4F, HF, NaF, KF and LiF, is investigated using cyclic voltammetry, chronoamperometry, atomic absorption spectroscopy and scanning electron microscopy. In addition to direct dissolution of nickel and dissolution through the oxide layer, a new mode of dissolution of NiF2 film as NiF3 − and NiF4 2− through complex formation is proposed. This process is significantly influenced by the alkali metal fluorides. On a comparative basis the stability of NiF2 decreases in the order NH4F > HF > KF > LiF.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 29 July 1998 / Accepted: 3 November 1998
Rights and permissions
About this article
Cite this article
Noel, M., Santhanam, R. & Chidambaram, S. The influence of monovalent cations on the stability of electrochemically formed nickel fluoride films. J Solid State Electrochem 3, 239–244 (1999). https://doi.org/10.1007/s100080050153
Issue Date:
DOI: https://doi.org/10.1007/s100080050153