Abstract
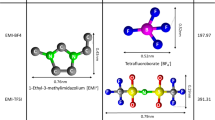
A potential new family of adaptable, ecologically acceptable solvent systems are ionic liquids (ILs). This study is the first to offer a new method for using imidazolium-based ILs as affordable, anti-corrosion electrolytes for activated carbon-based electrochemical capacitors (ECs). In this context, the ILs 1-hexyl 3-methylimidazolium bromide ([HMIM]Br) and 1-ethyl 3-methylimidazolium ethyl sulfate ([EMIM]ES) were synthesized. In the region of ambient temperature up to 105 °C, significant features including viscosity, electrical conductivity, and electrochemical tests were investigated. The symmetric ECs in imidazolium-based electrolytes, which display exceptional electrochemical stability at a maximum voltage of up to 3.5 V, deliver high energy. At room temperature, the specific capacitance of the electrolyte containing IL [HMIM]Br drops to 87 F g−1 (with an energy density of 37 Wh kg−1) from 174 F g−1 (with an energy density of 74 Wh kg−1) under 105 °C. In addition, the supercapacitor made using IL [HMIM]Br retained 90.6% of its capacity up to 10,000 cycles at 105 °C. The findings have significant ramifications for extending the use of eco-friendly, non-toxic, and affordable electrolytes for high-performance ECs with broad voltage windows and improved longevity.











Similar content being viewed by others
References
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science 321(5889):651–652
Pettersson F, Keskinen J, Remonen T, von Hertzen L, Jansson E, Tappura K, Zhang Y, Wilén C-E, Österbacka R (2014) Printed environmentally friendly supercapacitors with ionic liquid electrolytes on paper. J Power Sources 271:298–304
Zhou Y, Qi H, Yang J, Bo Z, Huang F, Islam MS, Lu X, Dai L, Amal R, Wang CH (2021) Two-birds-one-stone: multifunctional supercapacitors beyond traditional energy storage. Energy Environ Sci 14(4):1854–1896
Liu B, Wang X, Chen Y, Xie H, Zhao X, Nassr AB, Li Y (2023) Honeycomb carbon obtained from coal liquefaction residual asphaltene for high-performance supercapacitors in ionic and organic liquid-based electrolytes. J Energy Storage 68:107826
Zhang LL, Zhou R, Zhao X (2010) Graphene-based materials as supercapacitor electrodes. J Mater Chem 20(29):5983–5992
Fic K, Płatek A, Piwek J, Menzel J, Ślesiński A, Bujewska P, Galek P, Frąckowiak E (2019) Revisited insights into charge storage mechanisms in electrochemical capacitors with Li2SO4-based electrolyte. Energy Storage Mater 22:1–14
Zhang X, Wang Y, Yuan X, Shen Y, Lu Z, Wang Z (2022) Adaptive dynamic surface control with disturbance observers for battery/supercapacitor-based hybrid energy sources in electric vehicles, IEEE Trans Transp Electrification
Lu C, Zhou H, Li L, Yang A, Xu C, Ou Z, Wang J, Wang X, Tian F (2022) Split-core magnetoelectric current sensor and wireless current measurement application. Measurement 188:110527
Li M, Wang C, Chen Z, Xu K, Lu J (2020) New concepts in electrolytes. Chem Rev 120(14):6783–6819
Borodin O, Ren X, Vatamanu J, von Wald Cresce A, Knap J, Xu K (2017) Modeling insight into battery electrolyte electrochemical stability and interfacial structure. Acc Chem Res 50(12):2886–2894
Lu M (2013) Supercapacitors: materials, systems, and applications. John Wiley & Sons
Feng J, Wang Y, Xu Y, Sun Y, Tang Y, Yan X (2021) Ion regulation of ionic liquid electrolytes for supercapacitors. Energy Environ Sci 14(5):2859–2882
Zhang X, Tang Y, Zhang F, Lee CS (2016) A novel aluminum–graphite dual-ion battery. Adv Energy Mater 6(11):1502588
Mirzaei-Saatlo M, Asghari E, Shekaari H, Pollet B, Vinodh R (2023) Performance of ethanolamine-based ionic liquids as novel green electrolytes for the electrochemical energy storage applications. Electrochimica Acta 143499
Wang M, Jiang C, Zhang S, Song X, Tang Y, Cheng H-M (2018) Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat Chem 10(6):667–672
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7(11):845–854
Pech D, Brunet M, Taberna P-L, Simon P, Fabre N, Mesnilgrente F, Conédéra V, Durou H (2010) Elaboration of a microstructured inkjet-printed carbon electrochemical capacitor. J Power Sources 195(4):1266–1269
Earle MJ, Seddon KR (2000) Ionic liquids. Green solvents for the future. Pure Appl Chem 72(7):1391–1398
Wasserscheid P, Keim W (2000) Ionic liquids—new “solutions” for transition metal catalysis. Angew Chem Int Ed 39(21):3772–3789
Du S, Yin J, Xie H, Sun Y, Fang T, Wang Y, Li J, Xiao D, Yang X, Zhang S (2022) Auger scattering dynamic of photo-excited hot carriers in nano-graphite film. Appl Phys Lett 121(18)
Welton T (1999) Room-temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99(8):2071–2084
Hough WL, Rogers RD (2007) Ionic liquids then and now: from solvents to materials to active pharmaceutical ingredients. Bull Chem Soc Jpn 80(12):2262–2269
Minami I (2009) Ionic liquids in tribology. Molecules 14(6):2286–2305
Kim KS, Choi S, Demberelnyamba D, Lee H, Oh J, Lee BB, Mun SJ (2004) Ionic liquids based on N-alkyl-N-methylmorpholinium salts as potential electrolytes. Chem Commun (7):828–829
Greaves TL, Drummond CJ (2008) Protic ionic liquids: properties and applications. Chem Rev 108(1):206–237
Greaves TL, Weerawardena A, Fong C, Krodkiewska I, Drummond CJ (2006) Protic ionic liquids: solvents with tunable phase behavior and physicochemical properties. J Phys Chem B 110(45):22479–22487
Yoshizawa M, Xu W, Angell CA (2003) Ionic liquids by proton transfer: vapor pressure, conductivity, and the relevance of Δp K a from aqueous solutions. J Am Chem Soc 125(50):15411–15419
Xu W, Angell CA (2003) Solvent-free electrolytes with aqueous solution-like conductivities. Science 302(5644):422–425
Liu Y, Qin J, Lu L, Xu J, Su X (2023) Enhanced microwave absorption property of silver decorated biomass ordered porous carbon composite materials with frequency selective surface incorporation. Int J Miner Metall Mater 30(3):525–535
Timperman L, Galiano H, Lemordant D, Anouti M (2011) Phosphonium-based protic ionic liquid as electrolyte for carbon-based supercapacitors. Electrochem Commun 13(10):1112–1115
Qiu C, Jiang L, Gao Y, Sheng L (2023) Effects of oxygen-containing functional groups on carbon materials in supercapacitors: a review. Mater Des 111952
Lee AA, Vella D, Perkin S, Goriely A (2015) Are room-temperature ionic liquids dilute electrolytes? J Phys Chem Lett 6(1):159–163
Lewandowski A, Świderska-Mocek A (2009) Ionic liquids as electrolytes for Li-ion batteries—an overview of electrochemical studies. J Power Sources 194(2):601–609
Li H, Wang Y, Jiang F, Li M, Xu Z (2023) A dual-function [Ru (bpy) 3] 2+ encapsulated metal organic framework for ratiometric Al 3+ detection and anticounterfeiting application. Dalton Trans 52(12):3846–3854
Ashassi-Sorkhabi H, Kazempour A, Moradi-Alavian S, Asghari E, Lamb JJ (2023) 3D nanostructured nickel film supported to a conducting polymer as an electrocatalyst with exceptional properties for hydrogen evolution reaction. Int J Hydrogen Energy
Das HT, Dutta S, Das N, Das P, Mondal A, Imran M (2022) Recent trend of CeO2-based nanocomposites electrode in supercapacitor: a review on energy storage applications. J Energy Storage 50:104643
Yu H, Chen D, Li Q, Yan C, Jiang Z, Zhou L, Wei W, Ma J, Ji X, Chen Y (2023) In situ construction of anode–molecule interface via lone-pair electrons in trace organic molecules additives to achieve stable zinc metal anodes. Adv Energy Mater 2300550
Rawat S, Mishra RK, Bhaskar T (2022) Biomass derived functional carbon materials for supercapacitor applications. Chemosphere 286:131961
Yu H, Chen D, Ni X, Qing P, Yan C, Wei W, Ma J, Ji X, Chen Y, Chen L (2023) Reversible adsorption with oriented arrangement of a zwitterionic additive stabilizes electrodes for ultralong-life Zn-ion batteries, Energy Environ Sci
Fan Z, Ren J, Zhang F, Gu T, Zhang S, Ren R-P, Lv Y-K (2022) A flexible and self-healing supercapacitor based on activated carbon cloth/MnO2 composite. J Mater Sci 57(2):1281–1290
Loganathan NN, Perumal V, Pandian BR, Atchudan R, Edison TNJI, Ovinis M (2022) Recent studies on polymeric materials for supercapacitor development. J Energy Storage 49:104149
Liu W, Zhao Q, Yu H, Wang H, Huang S, Zhou L, Wei W, Zhang Q, Ji X, Chen Y (2023) Metallic particles-induced surface reconstruction enabling highly durable zinc metal anode. Adv Funct Mater 2302661
Mirheydari SN, Barzegar-Jalali M, Faraji S, Shekaari H, Martinez F, Jouyban A (2020) Volumetric and acoustic properties of ionic liquid, 1-hexyl-3-methylimidazolium bromide in 1-hexanol, 1-heptanol and 1-octanol at T=(298.15–328.15) K. Phys Chem Liquids 58(4):545–558
Shekaari H, Zafarani-Moattar MT, Faraji S, Mokhtarpour M (2020) Prediction of vapor pressure and density for nonaqueous solutions of the ionic liquid 1-ethyl-3-methylimidazolium ethyl sulfate using PC-SAFT equation of state. Fluid Phase Equilib 506:112320
Vila J, Ginés P, Pico J, Franjo C, Jiménez E, Varela L, Cabeza O (2006) Temperature dependence of the electrical conductivity in EMIM-based ionic liquids: evidence of Vogel–Tamman–Fulcher behavior. Fluid Phase Equilib 242(2):141–146
Wang W, Sabugaa M, Chandra S, Asmara YP, Abd Alreda B, Ulloa N, Elmasry Y, Kadhim MM (2023) Choline chloride-based deep eutectic solvents as electrolytes for wide temperature range supercapacitors. J Energy Storage 71:108141
Dou Q, Lei S, Wang D-W, Zhang Q, Xiao D, Guo H, Wang A, Yang H, Li Y, Shi S (2018) Safe and high-rate supercapacitors based on an “acetonitrile/water in salt” hybrid electrolyte. Energy Environ Sci 11(11):3212–3219
Zhong M, Tang QF, Zhu YW, Chen XY, Zhang ZJ (2020) An alternative electrolyte of deep eutectic solvent by choline chloride and ethylene glycol for wide temperature range supercapacitors. J Power Sources 452:227847
Liu W, Zhao C, Zhou Y, Xu X (2022) Modeling of vapor-liquid equilibrium for electrolyte solutions based on COSMO-RS interaction. J Chem 2022:1–13
Mahanta U, Choudhury S, Venkatesh RP, SarojiniAmma S, Ilangovan S, Banerjee T (2019) Ionic-liquid-based deep eutectic solvents as novel electrolytes for supercapacitors: COSMO-SAC predictions, synthesis, and characterization. ACS Sustain Chem Eng 8(1):372–381
Wong SI, Lin H, Ma T, Sunarso J, Wong BT, Jia B (2022) Binary ionic liquid electrolyte design for ultrahigh-energy density graphene-based supercapacitors. Mater Rep Energy 2(2):100093
Ortega PF, Santos Jr GA, Trigueiro JP, Silva GG, Quintanal N, Blanco C, Lavall RL, Santamaría R (2020) Insights on the behavior of imidazolium ionic liquids as electrolytes in carbon-based supercapacitors: an applied electrochemical approach. J Phys Chem C 124(29):15818–15830
Sillars FB, Fletcher SI, Mirzaeian M, Hall PJ (2012) Variation of electrochemical capacitor performance with room temperature ionic liquid electrolyte viscosity and ion size. Phys Chem Chem Phys 14(17):6094–6100
Thangavel R, Kannan AG, Ponraj R, Thangavel V, Kim D-W, Lee Y-S (2018) High-energy green supercapacitor driven by ionic liquid electrolytes as an ultra-high stable next-generation energy storage device. J Power Sources 383:102–109
Kurig H, Vestli M, Jänes A, Lust E (2011) Electrical double layer capacitors based on two 1-ethyl-3-methylimidazolium ionic liquids with different anions. Electrochem Solid-State Lett 14(8):A120
Fic K, Gorska B, Bujewska P, Béguin F, Frackowiak E (2019) Selenocyanate-based ionic liquid as redox-active electrolyte for hybrid electrochemical capacitors. Electrochim Acta 314:1–8
Mahanta U, Venkatesh RP, Sujatha S, Ilangovan S, Banerjee T (2019) Imidazolium based ionic liquids as electrolytes for energy efficient electrical double layer capacitor: insights from molecular dynamics and electrochemical characterization. J Solution Chem 48:1119–1134
Maiti S, Pramanik A, Mahanty S (2015) Influence of imidazolium-based ionic liquid electrolytes on the performance of nano-structured MnO 2 hollow spheres as electrochemical supercapacitor. RSC Adv 5(52):41617–41626
Mohsenipour A, Mozaffarian M, Pazuki G, Naji L (2022) Fabrication of high performance supercapacitors based on ethyl methyl imidazolium bis (trifluoromethylsulfonyl) imide (EMIMTFSI)-decorated reduced graphene oxide (rGO). J Alloy Compd 892:162093
Chen X, Han J, Lv X, Lv W, Pan Z, Luo C, Zhang S, Lin Q, Kang F, Yang Q-H (2019) Dense yet highly ion permeable graphene electrodes obtained by capillary-drying of a holey graphene oxide assembly. J Mater Chem A 7(20):12691–12697
Song Z, Duan H, Li L, Zhu D, Cao T, Lv Y, Xiong W, Wang Z, Liu M, Gan L (2019) High-energy flexible solid-state supercapacitors based on O, N, S-tridoped carbon electrodes and a 3.5 V gel-type electrolyte. Cheml Eng J 372:1216–1225
Acknowledgements
The authors acknowledge the facilities from their universities.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Adel, H., Mohealdeen, S.M. et al. Application of imidazolium-based ionic liquids as electrolytes for supercapacitors with superior performance at a wide temperature range. J Solid State Electrochem (2023). https://doi.org/10.1007/s10008-023-05763-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10008-023-05763-9