Abstract
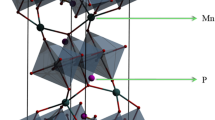
Transition metal Mn ions are highly promising cathode dopant materials. Due to the introduction of Mn ions, as lithium ions are deintercalated, Mn2+ will be transformed into Mn3+. This situation will lead to a severe Jahn-Teller effect, causing significant local lattice distortion and greatly reducing electrochemical stability. This article utilizes first-principles calculations to investigate the doping of Mg, Co, and V to weaken Jahn-Teller effect in LiMn0.5Fe0.5PO4 cathode. The oxidation-reduction processes of three doped models were analyzed, and the electronic structure and charge transfer amount between the Mn ion and the O ion were calculated for each. It was found that, when Mg ions are doped into the crystal, Mn ions will stabilize as Mn2+, thereby weakening the Jahn-Teller effect. However, the addition of V and Co will not alter the Jahn-Teller effect. The differential charge density and partial density of states (PDOS) were also calculated. It was found that only the doping of Mg ions can enable the material to achieve the lowest energy and the smallest volume change rate, which attribute to weaken the Jahn-Teller effect. Only doping with V and Co ions can achieve the highest lithium removal voltage, increasing the average lithium removal voltage from 4.22 to 4.42 V. Mechanical performance calculations show that the structures with two types of doped Mg ion are prone to shear deformation and cannot improve the ductility of the material. Additionally, it was found that the migration barrier of the three doped models was reduced to varying degrees, which is beneficial for the transition of lithium ions. Moreover, the diffusion coefficient of lithium ions also increased by 1–4 orders of magnitude.




Similar content being viewed by others
References
Padhi AK, Nanjundaswamy KS, Goodenough JB (1997) Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. J Electrochem Soc 144:1188
Ni L, Zhang S, Di A, Deng W, Zou G, Hou H et al (2022) Challenges and strategies towards single-crystalline Ni-Rich layered cathodes. Energy Materials 12:2201510
Ni L, Chen H, Guo S, Dai A, Gao J, Yu L et al (2023) Enabling structure/interface regulation for high performance Ni‐Rich cathodes. Funct Mater 2307126
Ni L, Chen H, Gao J, Mei Y, Wang H, Zhu F et al (2023) Calcium-induced pinning effect for high-performance Co-free Ni-rich NMA layered cathode. Energy 115:108743
Goodenough JB, Park K-S (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135:1167–1176
Yu H, Zhou H (2013) High-energy cathode materials (Li2MnO3–LiMO2) for lithium-ion batteries. J Phys Chem Lett 4:1268–1280
Manthiram A (2020) A reflection on lithium-ion battery cathode chemistry. Nat Commun 11:1550
Liang Y, Zhao CZ, Yuan H, Chen Y, Zhang W, Huang JQ et al (2019) A review of rechargeable batteries for portable electronic devices. InfoMat 1:6–32
Li F, Li J, Zhu F, Liu T, Xu B, Kim T-H et al (2019) Atomically intimate contact between solid electrolytes and electrodes for Li batteries. Matter 1:1001–1016
Wang C, Yuan X, Tan H, Jian S, Ma Z, Zhao J et al (2021) Three-dimensional carbon-coated LiFePO4 cathode with improved Li-ion battery performance. Coatings 11:1137
Yan X, Yang Y, Li C, Liu J, Wang J, Xi F et al (2022) The synthesis of LiFePO4/C with polyaniline as coated carbon source and sucrose as reducing carbon source. Ionics 28:1559–1571
Cholsuk C, Suwanna S, Sukkabot W, Busayaporn W, Suwanna P (2020) First-principles study of effects of combined ti supervalent cations and lithium ion vacancies doping on crystal and electronic structures and conductivity in LiFePO4. Eng Mater 861:277–283
Cui Z, Guo X, Ren J, Xue H, Tang F, La P et al (2021) Enhanced electrochemical performance and storage mechanism of LiFePO4 doped by Co, Mn and S elements for lithium-ion batteries. Acta 388:138592
Nwachukwu IM, Nwanya AC, Ekwealor A, Ezema FI (2022) Recent progress in Mn and Fe-rich cathode materials used in Li-ion batteries. J Energy Storage 54:105248
Daigle J-C, Rochon S, Asakawa Y, Fleutot B, Mallet C, Amouzegar K et al (2021) High performance LiMnFePO4/Li4Ti5O12 full cells by functionalized polymeric additives. Advances 2:253–260
Liu S, Wang B, Zhang X, Zhao S, Zhang Z, Yu H (2021) Reviving the lithium-manganese-based layered oxide cathodes for lithium-ion batteries. Matter 4:1511–1527
Bedoya-Lora FE, Vásquez-Salgado V, Vásquez FA, Calderón JA (2023) Stable V-doped LiMnPO4/C cathode material for Li-ion batteries produced by a fast and facile microwave-assisted synthesis. J Alloys Compd 938:168445
Nie Z, Ouyang C, Chen J, Zhong Z, Du Y, Liu D et al (2010) First principles study of Jahn-Teller effects in LixMnPO4. State Communications 150:40–44
Oukahou S, Maymoun M, Elomrani A, Sbiaai K, Hasnaoui A (2022) Enhancing the electrochemical performance of olivine LiMnPO4 as cathode materials for Li-Ion batteries by Ni–Fe codoping. Appl Energy Mater 5:10591–10603
Wani TA, Suresh G (2021) A comprehensive review of LiMnPO4 based cathode materials for lithium-ion batteries: current strategies to improve its performance. J Energy Storage 44:103307
He W, Guo W, Wu H, Lin L, Liu Q, Han X et al (2021) Challenges and recent advances in high capacity Li-rich cathode materials for high energy density lithium-ion batteries. Materials 33:2005937
Feng Y, Zhou L, Ma H, Wu Z, Zhao Q, Li H et al (2022) Challenges and advances in wide-temperature rechargeable lithium batteries. Energy Environ Sci 15:1711–1759
Armstrong AR, Bruce PG (1996) Synthesis of layered LiMnO2 as an electrode for rechargeable lithium batteries. Nature 381:499–500
Rossouw M, Thackeray M (1991) Lithium manganese oxides from Li2MnO3 for rechargeable lithium battery applications. Mater Res Bull 26:463–473
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758
Liu Y, Gu Y, Zeng H, Zheng J, Pan F (2019) Role of superexchange interactions on the arrangement of Fe and Mn in LiMnxFe1–xPO4. J Phys Chem C 123:17002–17009
Assat G, Tarascon J-M (2018) Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Energy 3:373–386
Huang Z-F, Meng X, Wang C-Z, Sun Y, Chen G (2006) First-principles calculations on the Jahn-Teller distortion in layered LiMnO2. J Power Sources 158:1394–1400
Mishra S, Ceder G (1999) Structural stability of lithium manganese oxides. Phys Rev B 59:6120
Ceder G, Mishra S (1999) The stability of orthorhombic and monoclinic-layered LiMnO2. Electrochem Solid-State Lett 2:550
Ohzuku T, Kato J, Sawai K, Hirai T (1991) Electrochemistry of manganese dioxide in lithium nonaqueous cells: IV. Jahn‐Teller deformation of MnO6 - Octahedron in LixMnO2. J Electrochem Soc 138:2556
Dou J-Q, Kang X-Y, Tuerdi W, Hua N, Han Y (2012) The first principles and experimental study on Mn-doped LiFePO4
Liu L, Chen G, Du B, Cui Y, Ke X, Liu J et al (2017) Nano-sized cathode material LiMn0.5Fe0.5PO4/C synthesized via improved sol-gel routine and its magnetic and electrochemical properties. Acta 255:205–211
Hao G, Lai Q, Zhang H (2021) Nanostructured Mn-based oxides as high-performance cathodes for next generation Li-ion batteries. J Energy Chem 59:547–571
Maxisch T, Ceder G (2006) Elastic properties of olivine LixFePO4 from first principles. Phys Rev B 73:174112
Wu Z-J, Zhao E-J, Xiang H-P, Hao X-F, Liu X-j, Meng J (2007) Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys Rev B 76:054115
Shu-Lin Z, Jia-Hao Q, Wen-Wei L, Mu-Sheng W (2021) First-principles study of properties of rare-earth-doped LiFePO4. Physica Sinica 70
Zhang D, Wang J, Dong K, Hao A (2018) First principles investigation on the elastic and electronic properties of Mn Co, Nb, Mo doped LiFePO4. Mater Sci 155:410–415
Gaddam V, Das D, Jung T, Jeon S (2021) Ferroelectricity enhancement in Hf0.5Zr0.5O2 based tri-layer capacitors at low-temperature (350 °C) annealing process. Electron Device Letters 42:812–815
Tong Z, Bazri B, Hu S-F, Liu R-S (2021) Interfacial chemistry in anode-free batteries: challenges and strategies. J Mater Chem A 9:7396–7406
Lee W, Kim J, Yun S, Choi W, Kim H, Yoon W-S (2020) Multiscale factors in designing alkali-ion (Li, Na, and K) transition metal inorganic compounds for next-generation rechargeable batteries. Energy Environ Sci 13:4406–4449
Zhang M, Kitchaev DA, Lebens-Higgins Z, Vinckeviciute J, Zuba M, Reeves PJ et al (2022) Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Rev Mater 7:522–540
Xiao Y, Zhang FC, Han JI (2016) Synthesis, characterization and lithium-ion migration dynamics simulation of LiFe1–x Tx PO4 (T = Mn, Co, La and Ce) doping cathode material for lithium-ion batteries. Physics A 122:1–12
Lanjan A, Ghalami Choobar B, Amjad-Iranagh S (2020) Promoting lithium-ion battery performance by application of crystalline cathodes LixMn1−zFezPO4. J Solid State Electrochem 24:157–171
Funding
This research was financially supported by the National Natural Science Foundation of China (NSFC, Nos. 52275095 and 22278080); Fujian Province Key Technology Innovation Tackling and Industrialization Project (2023G040).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lv, Z., Li, M., Lin, J. et al. First-principles study on LiMn0.5Fe0.5PO4 doping to decrease the Jahn-Teller effect. J Solid State Electrochem 28, 577–587 (2024). https://doi.org/10.1007/s10008-023-05705-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05705-5