Abstract
The corrosion behavior of electrodeposited nickel-based coatings with W, Mo, and TiOx as potential catalytic material for hydrogen evolution reaction (HER) in highly aggressive media, 0.5 M H2SO4 and 6 M KOH, is presented. A wide range of electrochemical and spectroscopic methods have been used, such as linear sweep voltammetry, open-circuit potential (OCP), electrochemical impedance spectroscopy (EIS), X-ray diffraction (XRD), X-ray fluorescence spectroscopy (XRF), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), and energy-dispersive spectroscopy (EDS) to characterize the coatings before and after a residence time of 336 h in the model corrosion environment. The NiW alloy and the NiWTiOx composite coating demonstrate the best corrosion resistance in an acidic environment due to the stable tungstate phase formation (H0.33O3W and H2O4W) on the surface. In alkaline environments, all the systems studied show enviable resistance, but the alloys containing molybdenum stand out.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common methods used to produce hydrogen is water splitting. In order to increase the efficiency of the process, it is necessary to create new electrode materials having high catalytic activity and low overvoltage with respect to the partial electrode reactions of hydrogen and oxygen evolution, as well as good corrosion resistance in the working environment. The aim is that these materials can replace the costly noble metals currently in use by approximating their characteristics as closely as possible.
Some of the most common electrode materials are nickel-based, correlated with metals of the iron group [1,2,3,4] or from baths containing particles such as Ti, TiO2, NiO, and others [5,6,7]. The resulting composites have a more developed, rough, or porous surface with good catalytic activity. An important requirement for the materials is to possess good corrosion resistance in the working environment. In recent years, the interest in electrolytically deposited binary nickel alloy coatings with tungsten or molybdenum as well as ternary ones of the Ni-Fe-W [8], Ni-Mo-W [2, 9], and Ni-Cr-Mo type [10] has been growing. The corrosion resistance of such coatings has been investigated in terms of the type and pretreatment of the substrate, their structure [11], the percentage of W or Mo in the alloy [12, 13], the surface morphology, the grain size [14, 15], the type of crystallographic texture [8, 12, 16], and the particular corrosion environment [12, 17, 18].
Increasing tungsten content (0–26%) in Ni-W alloys results in amorphous alloy coatings with many cracks and surface defects, which worsens corrosion treatments. In the case of crystalline ones, however, increasing the percentage of tungsten content leads to the formation of a surface oxide film that enhances corrosion resistance [16]. This is due to preferential dissolution of nickel and formation of a tungsten-rich film on the electrode surface, which inhibits further corrosion [19]. Another factor that affects the corrosion behavior of Ni-W and Ni-Mo alloys is the grain size [14, 15, 20]. It has been found that in an alkaline medium with decreasing grain size, corrosion resistance increases while in an acidic medium, it leads to decrease the corrosion resistance [12]. Casciano and Benevides [17] performed a comparative study with electrodeposited Ni-W and Ni-Mo-W coatings to evaluate the influence of Mo content on corrosion resistance in NaCl solution. They found that the triple alloy gave a lower anodic current, i.e., had better corrosion resistance as a result of the formation of a secondary oxide layer formed on the already existing primary oxide layer.
Baghery et al. [21] have investigated Ni-TiO2 nanocomposite coatings in terms of their corrosion and wear resistance. By varying the TiO2 particle content in the electrolyte, the current density, and the stirring rate of the electrolyte, they obtained coatings with different compositions. They found that as the TiO2 particle content of the coating increased, its microhardness, wear resistance, and polarization resistance increased, while the corrosion current decreased and the corrosion potential shifted to more positive values. Li et al. [22] have investigated the influence of current density and deposition time on the microstructure and corrosion resistance of pulse electroplating Ni-W/TiN nanocomposite coatings. They report that the composites obtained by this method are uniform, compact, and crack-free. They found that the incorporation of TiN in the Ni-W matrix filled the defects (cracks), resulting in better corrosion resistance in 3.5% NaCl.
In our previous studies, we have demonstrated the good catalytic activity for HER of alloy and composite nickel coatings with W, Mo, and TiOx [23,24,25]. In this work, a comparative study of the corrosion resistance of our catalytically active nickel-based alloy and composite coating in highly aggressive media, 0.5M H2SO4 and 6M KOH, is presented. The corrosion behavior of the coatings before and after a 336-h (2 weeks) residence time in the model media is investigated by classical electrochemical methods. Impedance spectroscopy is used for quality elucidation of the processes occurring at the electrode-electrolyte interface.
Experimental
All coatings were deposited under a potentiostatic condition on a copper plate substrate with a geometric area of 2 cm2. The composition of the electrolytes and the electrodeposition conditions are presented in Table S1 [2]. The deposition and electrochemical measurements were performed in a conventional three-electrode cell. A platinized titanium mesh (TiPt mesh) was used as a counter-electrode and a saturated calomel electrode (SCE) as a reference electrode. Ti oxides were maintained in suspension by stirring for 150 min−1. These conditions produce a coating with a relatively uniform distribution of TiOx particles on the surface (Fig. S1).
The morphology and composition of the coatings were investigated using a scanning electron microscope JOEL JSM 6390 (SEM) equipped with an Oxford Instruments INCA x-sight energy-dispersive spectrometer (EDS). A FISHERSCOPE X-RAY, an X-ray fluorescence instrument (XRF), was used to determine the elemental composition and thickness of the materials. As shown in Fig. S2, measurements were carried out at five well-defined points on the electrode surface. The structure of the samples was examined using X-ray diffraction (XRD) analysis by a Philips PW 1050 diffractometer equipped with a copper anode X-ray tube and a secondary beam monochromator operating at 40 kV, 30 mA, with a step size of 0.05 (2Ө), and an exposure time of 4 s. The XPS measurements were performed in the analysis chamber of the electron spectrometer ESCALAB Mk II (VG Scientific) with a base pressure of ~ 5 × 10−8 Pa with the excitation radiations MgKα (hν = 1253.6 eV) and AlKα (hν = 1486.6 eV). All spectra were calibrated by using C1s peak at 285.0 eV as a reference.
The corrosion studies were carried out in two model media (0.5M H2SO4 and 6M KOH solutions) at room temperature before and after a residence time of 336 h by electrochemical impedance spectroscopy (EIS) and polarization resistance (Rp) methods. EIS measurements were performed over the frequency range of 50 kHz to 10 mHz, with 10 points per decade, 10 mV sine wave amplitude, and at open-circuit potential using a potentiostat/galvanostat Gamry Interface 1000. The impedance spectra were analyzed using equivalent circuits. ZView 3.2c software was used for the nonlinear least squares fitting procedure. The polarization resistance was measured with potentiostat/galvanostat EG& PAR—Versastatt, SoftCorr 352. The potential was swept from −20 to + 20 mV vs. open-circuit potential at a scan rate of 1 mV/s. The polarization resistance was measured automatically using the Tafel slope method provided by corrosion software.
Results and discussion
Structural characterization of coatings as prepared
XRD patterns show that the studied coatings keep the fcc crystal structure of nickel, with W and/or Mo elements incorporated into the crystal lattice, resulting in a shift of the diffraction peaks to lower 2Ө angles relative to pure nickel (Fig. 1). This means that the formed fcc nickel alloys have a larger crystal lattice parameter relative to pure nickel (aNi=3.5209Å, aNiW=3.5760Å, aNiWTiOx=3.5730Å and aNiMo=3.5607Å, aNiMoW=3.5532Å). The crystallite size of the alloys (10–12 nm) is about 2 to 3 times smaller than that of pure nickel (27 nm). The reason for this on the one hand is that the insertion of atoms of different sizes leads to the formation of defects and makes it difficult to form large regions of coherent scattering, i.e., large crystallites. On the other hand, it is the more negative potential at which the alloys are deposited (−1100 mV for NiMo, NiMoW and −1200 mV for NiW, NiWTiOx) compared to pure Ni (−1000 mV).
The XPS spectra (Fig. 2) show that in addition to the metal phases, the corresponding metals are present in different valence states in oxides and hydroxides on the surface of the as prepared coatings (as-prepared—hereafter in the text denoted as fresh). In order to analyze the XPS in detail, a deconvolution of the high-resolution spectra of the individual elements Ni2p3/2 (Fig. 2a), Mo3d5/2 (Fig. 2b), W4f7/2 (Fig. 2c), and O1s (Fig. 2d) was used, as described in detail in [26]. In addition to the presence of the Ni2p3/2 component at ≈852.7 eV, corresponding to Ni0 in all spectra of the fresh coatings, there are also detected at 854 and 856 eV, which together with the satellites (at ≈861 and ≈865 eV) correspond to nickel of the second valence, in oxides/hydroxides, as well as mixed oxides/hydroxides (Fig. 2a). Tungsten-binding energies (BE) for W4f7/2 are 31.3 eV, 34 eV, and 35.6 eV for W0, WOx (x<3), and WO3, respectively, (Fig. 2c) and Mo (BE of Mo3d5/2: 227.6 eV, 229, 5, 230.6, and 233 eV for Mo0, MoO, MoOx (2<x<3), and MoO3) (Fig.
2b) are also in different valence states. The type of oxides can also be determined from the BE of oxygen (Fig. 2d). The BE of O2- in the M-O bond is about 530 eV (529.4 eV for NiO, 530.6 for WO3, and 531 eV for MoO3), that of OH- is in the range 531–532 eV; above 532 eV is due to H2O. The position of the strongest component of the oxygen peak at 531.8 eV means that mainly hydroxides such as Ni(OH)2, MOx(OH)y are formed on the surface. All data from the XPS analyses are presented in Table S2.
Corrosion behavior of the coatings in 0.5M H2SO4 solution
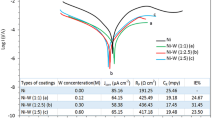
Potentiodynamic polarization curves of the studied fresh alloys including pure nickel are presented in Fig. 3. In the anodic region, three sections are observed: a region of hydrogen desorption or H oxidation process from −200 to −80 mV, a region of active dissolution of the alloys (increase in the anodic current density, −50 to +250 mV) and a region in which a tendency to passivation is observed (beyond about +250 mV), i.e., a decrease in the anodic current density. It can be seen in Fig. 3 that pure nickel coating shows the most positive corrosion potential (Ecorr) and the lowest corrosion current density (icorr), followed by NiW alloy (Table 1). Under anodic polarization, the nickel coating curve initially passes through a delayed dissolution region in the range of −160 to −50 mV, and then a very high dissolution current density region is present—practically the highest of all samples examined in this environment. The other systems are characterized by very similar values of corrosion current densities and corrosion potentials and show better corrosion treatments compared to pure nickel, i.e., lower anodic current of about 1–2 orders of magnitude. The most difficult anodic dissolution in this environment is observed for the NiWTiOx and NiW alloys, where broad passive regions of about 700 mV and current densities of about 1 mA dm−2 are recorded at potentials beyond about +250 mV. The decrease in anode currents is most likely a consequence of the formation of corrosion products on the surface, which block the intensive dissolution of alloys to some extent.
The fresh samples were immersed in the model medium for 2 weeks to follow the corrosion behavior of the alloys with time. The layer thickness and the change of the components in the alloys were analyzed by X-ray fluorescence analysis (Table 2) at the beginning and end of the period. The measurements as shown in Fig. S2 were taken at five points on the electrode surface, and the data obtained were averaged.
As can be seen from the table, the pure nickel coating is substantially dissolved. The least pronounced dissolution is detected for the NiW alloy, with no significant change in layer thickness observed before and after treatment in the model medium. For the NiWTiOx layers, a decrease in the percentage of nickel content as well as some thinning of the layer was indicated. The coatings with molybdenum co-deposition are almost completely dissolved. In general, for most alloys in this environment, a predominant dissolution of nickel and enrichment of the alloy of the remaining elements is observed. The XRF data obtained largely correspond with those from polarization curves.
Figure 4 shows the SEM images of the coatings before and after treatment in 0.5M H2SO4. A typical globular structure is observed for the nickel coating. In H2SO4 acid, the nickel layer dissolved almost completely (from about 7-μm thickness to 1–3 μm after 2 weeks of residence). The coating residue is a porous layer with a pore diameter of about 1 μm. The copper (68.8 wt.% Cu, 31.2 wt.% Ni) is highly visible in the pore depth with oxygen completely absent (Table S3). The surface morphology of NiW does not change significantly. Dissolution of the layer by about 1–2 μm is found (Table 2), with the W content increasing at the expense of Ni; i.e., Ni dissolves and W remains on the surface (fiber crystals from SEM). It is known that corrosion of NiW alloys in H2SO4 under OCP occurs by preferential dissolution of Ni from the surface layer and depending on how long the process continues, the more the surface becomes enriched in W [19]. The particle embedding in the coating is observed for NiWTiOx. Visibly after dissolution, the surface is relatively enriched in titanium oxides, which is an indication of dissolution of the NiW layer on them. Again the fiber crystals characteristic of NiW are observed. NiMo dissolves completely in the acid, more so than pure Ni (Table 2). On the surface (Fig. 4), white globules rich in O2, Mo, and Cu are observed, with O2 contents up to 20 wt.%. The NiMoW coating is also completely dissolved leaving a highly fractured layer on the surface consisting of 20 wt% O2, 35%Cu, 25% Mo, and 20% W and lacking Ni (Table S3).
Figure 5 presents the polarization resistance values of these same coatings measured after standing in the model corrosion medium (0.5 M H2SO4) for a period of 336 h (2 weeks). Alloys containing molybdenum showed the lowest polarization resistance (between 3 and 7 ohms) in the initial time. The presence of even a small amount of tungsten (about 3%) in molybdenum alloys significantly increases the polarization resistance after 1 week. But considering that the layer is almost dissolved (after 2 weeks), it is probably a film of corrosion products of Mo, W, and Cu.
For the NiW coatings, the highest Rp was observed at the initial time point, which increased after the first week and remained almost constant at around 500 ohms at the end of the period. For the NiW composite coating with incorporated non-stoichiometric titanium oxides, a lower polarization resistance is observed at the initial time point compared to the pure nickel and NiW coatings, but after about 336 h of residence in the model corrosion environment, it shows the best result. It is known that for nanocrystalline Ni-W alloys [12], the corrosion rate depends on the tungsten content, which promotes the formation of a corrosion-resistant oxide film, and the volume of grain boundaries that serve as active surface sites for corrosion reaction. In acidic environments with decreasing grain size of nanocrystalline Ni-W alloys, the corrosion rate decreases due to film formation, in which case the dominant factor is the tungsten content. Our results can be explained by the fact that on the sample surface hardly soluble oxides, hydroxides and hydrates of tungsten are formed as a layer of corrosion products with barrier properties, which block the electrode surface and make subsequent dissolution difficult.
This claim is confirmed by the XRD and XPS analyses, with the H0.33O3W and H2O4W phases registered on the X-ray diffraction patterns of the NiW and NiWTiOx coatings (Figs. 6 and S3).
Mo and W are in varying degrees of oxidation between 4+ and 6+ on the surface of the coating after 2 weeks of residence in H2SO4 (Fig. 7b and c, Table S2). The main oxygen peak for the pure Ni coating does not change its position (531.8 eV, Fig. 7d); i.e., only the ratio between Ni0 and Ni2+ (Fig. 7a) changes in favor of the latter as the dissolution of nickel proceeds through NiOHads [27]. These intermediate species are transformed into Ni(OH)2 after removal from the medium. The component with binding energy ≤ 530 eV that corresponds to O2- in NiO is almost absent. The slight decrease of BE O1s to about 530.6 eV is mainly associated with oxides/hydroxides of W and Mo, and the well-defined component of O1s at 532.5 eV, besides adsorbed water, can be attributed to the formation of MeO3.(H2O)x, where Me=Mo,W). It is assumed that when molybdenum is immersed in sulfuric acid, it is coated with Mo(III) products, representing a chemisorbed layer or a thin oxide-hydroxide layer [28]. Unlike molybdenum coatings and pure nickel, which dissolve in acidic media, tungstate coatings exhibit enviable corrosion resistance. This is due to the formation on the surface of a passivating layer of WOx and WO3.(H2O)x [29], preventing further dissolution of nickel. SEM and XRD results and polarization studies prove the above statement.
In the study of corrosion resistance of coatings, the equivalent circuits with one time constant (equivalent circuit 1, Fig. 8a) is most often used to determine Rct (R1) and the double-layer capacitance Cdl (represented by C1 or CPE1), and Rs is the electrolyte resistance. The constant phase element (CPE) is defined as Z=1/(T(jω)α), where 0<α<1and if α =1 then T=C. But this is ideally the situation. In most cases, the ac response reflects different processes, which can be represented by the more generalized equivalent circuit 2 (Fig. 8a). Depending on the processes, Z may include different elements or a combination of elements. Diffusion is represented by WD or CPE (α≤0.5), and polarization by C or CPE (α > 0.6). The various phenomena associated with charge transfer across phase boundaries, phase formation, etc. are represented by parallel coupled (RC) or (RCPE), and dissolution or relaxation by (LR), where L is the inductance. Equivalent circuits and Nyquist plots of Ni film—fresh and after 336 h in 0.5 M sulfuric acid—are shown in Fig. 8a and b, respectively. When Ni is immersed in 0.5 M sulfuric acid, active dissolution takes place with periodic cycles of surface passivation. The instability of Rp indicates the presence of activation-passivation cycles in the corrosion process [30]. This instability also affects the impedance studies. The impedance with two time constants is modeled by equivalent circuit 3 (Fig. 8a), in which the element Z is replaced by (R2CPE2) describing the low-frequency part. Clearly here the process is slower and is associated with the formation and resistivity of the OH- adsorbed layer according to Eq. (1) [27]:
NiOH+ can then react in two different ways. If oxidation of NiOH+ leads to Ni2+, there is no passivation of the metal, but rather active corrosion of Ni according to Eq. (3):
This process is described with the (LRL) elements reflecting the negative semicircle in the low-frequency part of the impedance after 336 h in H2SO4 (equivalent circuit 4, Fig. 8a).
However, oxidation of the intermediate can lead to the formation of NiO, and there is passivation of Ni as shown in Eq. (4) [31]:
But given that XPS yields no NiO (or very negligible) and EDS does not detect oxygen at all, the process is unlikely. Rather, one has to account for the formation of a highly porous layer (see Fig. 4) in the pores of which alkalinization increases. The increase in pH due to hydrogen evolution reaction it more difficult to dissolve the nickel in the pore depth, thus the nickel will dissolve preferentially from the surface. This may be the reason for the increase in Rp=R1+R2 after 336 h.
The co-deposition of W reduces the corrosion of the coating at OCP in sulfuric acid at pH=3 and changes the ac response [32]. On the Nyquist plot, present one semicircle and the tail at low frequency side. This tail is associated with diffusion, new layer formation, and nickel dissolution occurring simultaneously. And in this sense, Z should involve a combination of different elements in a complicated version of the transmittance line. We represented this part with (RCPE) for resistivity estimation. Lagarde et al. [32] suggest that the response at low frequencies cannot be attributed to a diffusion process and is probably related to an adsorption phenomenon. As can be seen, there is not much difference in the total resistance between pure nickel and NiW at the beginning. But definitely the formed layer after 336 h of standing in sulfuric acid has a capacitive behavior leading to the formation of a double electric layer. Due to its inhomogeneity, instead of using a capacitor, Cdl (capacitance of the double layer) is modeled with a single CPEdl, giving a slightly sloping line (equivalent circuit 5, Fig. 8a). This result perfectly corresponds to structural studies proving the presence of a stable tungstate phase on the surface. The behavior of NiWTiOx is similar, but in this case, one more (RCPE) must be added due to the additional interfacial boundary with TiOx (equivalent circuit 6, Fig. 8a). The slight bending of the low-frequency part suggests that there is also charge transfer.
Corrosion behavior of the coatings in 6 M KOH solution
From the curves in Fig. 9a, it can be seen that despite the differences in corrosion potentials, the corrosion current densities and anodic dissolution currents of all systems are very low. Again, the nickel coating exhibits the lowest corrosion current density. Most likely, upon anodic polarization, they go into a passive state as a result of the formation of insoluble corrosion products on the surface. It is also seen that almost all coatings undergo a section of accelerated anodic dissolution in the region immediately after the corrosion potential (−450 to −110 mV), followed by passive domains of low anodic current density (−100 to +200 mV). In addition, studies in alkaline media generally show the lowest dissolution currents in value compared to the other media. After the peak of the transition of nickel hydroxide to nickel oxyhydroxide (+200 to +300 mV), a sharp increase in current density is observed due to oxygen evolution. The shift of the corrosion potentials (Fig. 9a, Table S4) of nickel alloys in a strongly negative direction compared to pure Ni is related to the absorption of hydrogen or the formation of hydrides during their preparation [33]. Peaks of hydrogen desorption or H oxidation process are observed in the region of − 600 to −550 mV. This assertion is illustrated in Fig. 9b of the same alloys after treatment for 2 weeks in 6 M KOH and standing in air. The corrosion potentials are shifted significantly in the positive direction, and the hydrogen desorption or H oxidation process peak is absent. The most positive corrosion potentials are shown by the NiMo and NiWTiOx alloys.
From the data obtained by XRF analysis before and after treatment in 6M KOH (Table S5), it can be seen that the percentage content of the coating components and their thickness remained practically unchanged after the corrosion tests were carried out. SEM images also do not show any significant differences (Fig. S4). Only the surface in all alloys including nickel appears to be masked probably by the presence of a protective layer.
The polarization resistance data are presented in Fig. 10. At the initial time point (about 2 h after obtaining the coatings), no significant differences in polarization resistance values are observed. However, after the first week, a significant increase in Rp was registered for all coatings, after which a decrease in values was observed for Ni and NiWTiOx. Probably, in this highly alkaline environment, a stable film of oxides/hydroxides or mixed hydroxides is formed almost immediately, which block the surface and significantly lower the rate of corrosion processes. EDS analysis (Table S3) found an increase in O2 content to 11 wt.% compared to the freshly deposited coating of 0%. According to the Pourbaix diagram in 6 M KOH (pH above 14) [34], Ni, Mo, and W dissolved and formed HNiO2-, MoO42−, and WO42− ions, respectively. These ions are stable and probably adsorb to the surface, forming a protective layer of different species. For example, at pH > 8, W is assumed to be covered by a film of WO2 [35] at open circuit in the steady state. This film is oxidized to WO3 and subsequent interaction with OH− to WO42−, respectively. Another reason for film buildup may be the formation of poorly soluble compounds with K+ ions. It has been found [36] that K+ ions diffuse up to 660 nm in NiMo alloy. In this regard, the highest polarization resistance values of molybdenum alloys could be attributed to the greater film resistance of the mixed hydroxides compared to the other systems.
XRD patterns of the coatings did not register any other phases except the fcc Ni/NiMe phase (Me=W, Mo) after 2-week residence time in the alkaline media (Fig. S5). XPS analyses (Fig. 11) show that the surface of the coatings is oxidized. The location of the main oxygen peak O1s (Fig. 11d) around 531.4–531.6 eV suggests that the metals on the surface are present mainly as nickel hydroxides and/or mixed NiMeO(OH)x hydroxides. But considering also the Pourbaix potential-pH equilibrium diagram for nickel in 6 M KOH [34], it follows that some OH− bonding is also due to the formation of HNiO2− ions. A significant amount of potassium (see Table S3) is also recorded in the coating, which is a prerequisite for its incorporation into the hydroxides. A decrease in the Mo and W contents on the surface of the coatings is registered, which is due to the higher solubility of Mo and W in an alkaline medium [34].
As in both acid and alkaline solutions, the progress of the impedances is specific and is described by different combinations of R and CPE elements reflecting the redox, diffusion, and adsorption-desorption processes occurring on the surface of the coatings summarized in Fig. 8a. After 336 h of nickel coating in the alkaline solutions, the Nyquist plot (Fig. 12) can be modeled with a single (RCPE) element in combination with WD or CPE representing charge transfer (the semicircle) and diffusion or adsorption-desorption processes (the slope line), respectively (equivalent circuit 5, Fig. 8a). Surprisingly, Rct has a relatively low value (25 ohms) for nickel in 6 M KOH. The explanation may be related to the high pH, where in addition to the already formed hydroxide/oxyhydroxide layer, there is also a process of HNiO2- ion formation. The tailing can be explained by adsorption-desorption of HNiO2- ions and/or by diffusion limitations due to either diffusion of potassium ions or oxygen. For NiW and NiMo coatings, the behavior is similar to Ni and is described by the same equivalent circuit but different Rct. Again, the dominant process has diffusion character at low frequencies (Fig. 12). Hence, the ac response of NiWTiOx could be described with equivalent circuit 7, instead of equivalent circuit 6 (Fig. 8a). The strange pattern of impedance dependence of fresh NiMoW (Fig. S6) can be explained by adsorption/desorption of MoO42−, and relaxation processes consequently a protective layer is formed. After a residence time of 336 h in the alkaline media, this alloy shows the highest Rp value, which is confirmed by the Rp dependence (Fig. 10). Apparently, a sufficiently thick and dense film is obtained, resulting in an R2 resistance increase of more than 3 orders of magnitude after 336 h (equivalent circuit 3, Fig. 8a).
Conclusion
The coatings studied are characterized by the fcc crystal structure of nickel in which W and/or Mo are incorporated into the crystal lattice. These elements exhibit different valence states on the surface, from 4+ to 6+. When treated in 0.5 M sulfuric acid, NiW and NiWTiOx coatings show enviable corrosion resistance: high polarization resistance and low dissolution current densities. This fact is due to the formation of a stable tungstate phase (H0.33O3W and H2O4W) on the surface as fibre crystals, which blocks the electrode surface and makes subsequent dissolution difficult. In addition, impedance studies provide evidence for the simultaneous processes of diffusion, new layer formation, and nickel dissolution. In contrast to coatings with high tungsten content, the nickel and alloys with molybdenum dissolve almost completely in this medium. However, in 6 M KOH, all the coatings investigated exhibited significant corrosion resistance. Ni, Mo, and W were partially dissolved and HNiO2−, MoO42−, and WO42− ions were formed. These ions are stable and probably adsorb on the surface to form a protective layer of different species and/or mixed hydroxides NiMeO(OH)x, where Me = Mo or W. The alloys with molybdenum show the highest value of polarization resistance in 6 M KOH, which is due to the better film stability of the mixed hydroxides compared to the other systems. The presence of a diffusion component in the impedance results is evidence of the adsorption-desorption behavior of these ions for the formation of a protective layer.
References
Fan C, Piron DL, Sleb A, Paradis P (1994) Study of electrodeposited nickel-molybdenum, nickel-tungsten, cobalt-molybdenum, and cobalt-tungsten as hydrogen electrodes in alkaline water electrolysis. J Electrochem Soc 141:382–387. https://doi.org/10.1149/1.2054736
Rashkov R (2008) Synthesis and characterization of novel electrodeposited catalytic materials for hydrogen evolution reaction. Bulg Chem Commun 40:211–218 http://www.bcc.bas.bg/bcc_volumes/volume_40_number_3_2008/Volume_40_Number_3_2008_PDF/2814-RR.pdf
Halim J, Abdel-Karim R, El-Raghy S, Nabil M, Waheed A (2012) Electrodeposition and characterization of nanocrystalline Ni-Mo catalysts for hydrogen production. J Nanomater 2012:1–9. https://doi.org/10.1155/2012/845673
Arunsunai Kumar K, Paruthimal Kalaignan G, Muralidharan VS (2012) Pulse electrodeposition and characterization of nano Ni-W alloy deposits. Appl Surf Sci 259:231–237. https://doi.org/10.1016/j.apsusc.2012.07.024
Panek J, Serek A, Budniok A, Rówinski E, Łagiewka E (2003) Ni + Ti composite layers as cathode materials for electrolytic hydrogen evolution. Int J Hydrogen Energy 28:169–175. https://doi.org/10.1016/S0360-3199(02)00055-1
Bolat G, Mareci D, Iacoban S, Cimpoesu N, Munteanu C (2013) The estimation of corrosion behavior of NiTi and NiTiNb alloys using dynamic electrochemical impedance spectroscopy. J Spectroscopy 2013(1-2). https://doi.org/10.1155/2013/714920
Assis D, Wolynec S, Costa I (2006) Corrosion characterization of titanium alloys by electrochemical techniques. Electrochim Acta 51:1815–1819. https://doi.org/10.1016/j.electacta.2005.02.121
Sriraman KR, Ganesh Sundara Raman S, Seshadri SK (2007) Corrosion behaviour of electrodeposited nanocrystalline Ni–W and Ni–Fe–W alloys. Mater Sci Eng A 460–461:39–45. https://doi.org/10.1016/j.msea.2007.02.055
Arnaudova M, Avdeev G, Rashkov R, Mitov M, Zielonka A, Theodoridou E (2010) Electrodeposition and characterization of Ni-Mo and Ni-Mo-W layers on carbon fibers. 275-283. https://www.researchgate.net/publication/235736797
Lloyd AC, Noël JJ, McIntyre S, Shoesmith DW (2004) Cr, Mo and W alloying additions in Ni and their effect on passivity. Electrochim Acta 49:3015–3027. https://doi.org/10.1016/j.electacta.2004.01.061
Niedbała J (2015) Structure, morphology and corrosion resistance of Ni-Mo+PTh composite coatings. Bull Mater Sci 38:695–699. https://doi.org/10.1007/s12034-015-0922-z
Chianpairot A, Lothongkum G, Schuh CA, Boonyongmaneerat Y (2011) Corrosion of nanocrystalline Ni–W alloys in alkaline and acidic 3.5wt.% NaCl solutions. Corros Sci 53:1066–1071. https://doi.org/10.1016/j.corsci.2010.12.001
Hosseini MG, Teymourinia H, Farzaneh A, Khameneh-asl S (2016) Evaluation of corrosion, mechanical and structural properties of new Ni – W – PCTFE nanocomposite coating. Surf Coat Technol 298:114–120. https://doi.org/10.1016/j.surfcoat.2016.04.060
Wasekar NP, Verulkar S, Vamsi MVN, Sundararajan G (2019) Influence of molybdenum on the mechanical properties, electrochemical corrosion and wear behavior of electrodeposited Ni-Mo alloy. Surf Coat Technol 370:298–310. https://doi.org/10.1016/j.surfcoat.2019.04.059
Huang P-C, Hou K-H, Wang G-L, Chen M-L, Wang J-R (2015) Corrosion resistance of the Ni-Mo alloy coatings related to coating’s electroplating parameters. Int J Electrochem Sci 10:4972–4984
Alimadadi H, Ahmadi M, Aliofkhazraei M, Younesi SR (2009) Corrosion properties of electrodeposited nanocrystalline and amorphous patterned Ni–W alloy. Mater Des 30:1356–1361. https://doi.org/10.1016/j.matdes.2008.06.036
Casciano PNS, Benevides RL (2014) Corrosion resistance of electrodeposited Ni-Mo-W coatings. Int J Electrochem Sci 9:4413–4428. https://doi.org/10.1016/S1452-3981(23)08103-8
Makarova IV, Kharitonov DS, Dobryden IB, Chernik AA (2018) Corrosion behavior in acid and alkaline media of nickel coatings deposited at room temperature. Russ J Appl Chem 91:1441–1450. https://doi.org/10.1134/S1070427218090069
Obradović M, Stevanović J, Despić A, Stevanović R, Stoch J (2001) Characterization and corrosion properties of electrodeposited Ni-W alloys. J Serbian Chem Soc 66:899–912. https://doi.org/10.2298/jsc0112899o
Wang L, Zhang J, Gao Y, Xue Q, Hu L, Xu T (2006) Grain size effect in corrosion behavior of electrodeposited nanocrystalline Ni coatings in alkaline solution. Scr Mater 55:657–660. https://doi.org/10.1016/j.scriptamat.2006.04.009
Baghery P, Farzam M, Mousavi AB, Hosseini M (2010) Ni-TiO2 nanocomposite coating with high resistance to corrosion and wear. Surf Coatings Technol 204:3804–3810. https://doi.org/10.1016/j.surfcoat.2010.04.061
Li B, Li D, Chen W, Liu Y, Zhang J, Wei Y, Zhang W, Jia W (2019) Effect of current density and deposition time on microstructure and corrosion resistance of Ni-W/TiN nanocomposite coating. Ceram Int 45:4870–4879. https://doi.org/10.1016/j.ceramint.2018.11.184
Rashkov R, Atanassov N, Jannakoudakis A, Jannakoudakis P, Theodoridou E (2006) Structure and electrocatalytic activity of Ni–W thin films deposited on carbon fiber supports. J Electrochem Soc 153:C152. https://doi.org/10.1149/1.2163812
Mitov M, Hristova E, Hristov G, Rashkov R, Arnaudova M, Popov A (2009) Catalytic activity of NiW electrodeposits. Environ Chem Lett 7:249–253. https://doi.org/10.1007/s10311-008-0156-z
Rashkov R, Arnaudova M, Avdeev G, Zielonka A, Jannakoudakis P, Jannakoudakis A, Theodoridou E (2009) NiW/TiOx composite layers as cathode material for hydrogen evolution reaction. Int J Hydrogen Energy 34:2095–2100. https://doi.org/10.1016/j.ijhydene.2009.01.001
Bachvarov V, Arnaudova M, Lefterova E, Rashkov R (2022) Study of oxygen evolution reaction on iron group-based electrodeposited multicomponent catalysts in alkaline media. Part II: Surface investigations and corrosion durability. J Chem Technol Metall 57:1202–1211
Sato N, Okamoto G (1963) Anodic Passivation of nickel in sulfuric acid solutions. J Electrochem Soc 110:605. https://doi.org/10.1149/1.2425838
Bojinov M, Betova I, Raicheff R (1996) A model for the transpassivity of molybdenum in acidic sulphate solutions based on ac impedance measurements. Electrochim Acta 41:1173–1179. https://doi.org/10.1016/0013-4686(95)00468-8
Lillard R, Kanner GS, Buif DP (1998) The nature of oxide films on tungsten in acidic and alkaline solutions. J Electrochem Soc 145:2718–2725. https://doi.org/10.1149/1.1838704
Cruz MJ, Makarova IV, Kharitonov DS, Dobryden I, Chernik AA, Grageda M, Ushak S (2019) Corrosion properties of nickel coatings obtained from aqueous and nonaqueous electrolytes. Surf Interface Anal 51:943–953. https://doi.org/10.1002/sia.6683
Toloei AS, Stoilov V, Northwood DO (2014) An electrochemical impedance spectroscopy and potentiodynamic polarization study of the effect of unidirectional roughness on the corrosion of nickel. Int J Comput Methods Exp Meas 2:243–254. https://doi.org/10.2495/CMEM-V2-N3-243-254
Lagarde M, Billard A, Creus J, Feaugas X, Grosseau-Poussard JL, Touzain S, Savall C (2018) Electrochemical behavior of Ni e W alloys obtained by magnetron sputtering. Surf Coat Technol 352:581–590. https://doi.org/10.1016/j.surfcoat.2018.07.071
Vračar L, Conway BE (1990) Hydride formation at Ni-containing glassy-metal electrodes during the H2 evolution reaction in alkaline solutions. J Electroanal Chem Interfacial Electrochem 277:253–275. https://doi.org/10.1016/0022-0728(90)85106-F
Pourbaix M (1974) Atlas of electrochemical equilibria in aqueous solutions, 2nd edn. National association of corrosion engineers, Houston, Texas, p 77084
Heumann T, Stolica N (1971) The electrochemical behaviour of tungsten-II. The dissolution of tungsten in NaOH solutions. Electrochim Acta 16:1635–1646. https://doi.org/10.1016/0013-4686(71)85074-0
Wijten JHJ, Riemersma RL, Gauthier J, Mandemaker LDB, Verhoeven (Tiny) MWGM, Hofmann JP, Chan K, Weckhuysen BM (2019) Electrolyte effects on the stability of Ni−Mo cathodes for the hydrogen evolution reaction. ChemSusChem 12(3491):3500. https://doi.org/10.1002/cssc.201900617
Funding
This work is supported by the Bulgarian Ministry of Education and Science under National Roadmap for Scientific Infrastructure (CMD No. 354 of 29.06.2017) “Distributed infrastructure of centers for synthesis and characterization of new materials and conservation of archeological and ethnographic artefacts, INFRAMAT” (Contract D01-172/28.07.2022 г.) and the National Research Programme “Low Carbon Energy for the Transport and Households” (E+), grant agreement D01-214/2018.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arnaudova, M., Lefterova, E. & Rashkov, R. Corrosion behavior of electrodeposited nickel-based coatings with W, Mo, and TiOx. J Solid State Electrochem 28, 1657–1670 (2024). https://doi.org/10.1007/s10008-023-05696-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05696-3