Abstract
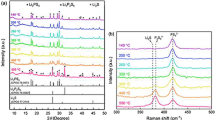
The solid electrolytes with a low melting temperature are promising for the all-solid-state lithium batteries because such electrolytes enable the battery fabrication without high-temperature sintering (for example, ~ 1000 °C for oxide materials). In this study, a series of LiOH-Li2SO4 systems with different LiOH/Li2SO4 ratios is fabricated by melting LiOH and Li2SO4 at 430 °C, and their ion conduction properties are investigated. The stoichiometric compounds are obtained in 2.2LiOH‧Li2SO4 without segregation of LiOH and Li2SO4, which is rather different from the previously known composition (3LiOH‧Li2SO4). The deviation from this LiOH/Li2SO4 fraction results in a segregation of LiOH or Li2SO4. The conductivities of 2.2LiOH‧Li2SO4 with 5 mol% of Li3BO3 are 1.9 × 10−6 and 6.0 × 10−3 S/cm at 25 and 150 °C, respectively. The all-solid-state batteries are fabricated by hot pressing the solid electrolyte with Li(Ni0.3Co0.6Mn0.1)O2 (cathode) and graphite (anode) at 250 °C under 150 MPa. The contacts of the solid electrolyte with Li(Ni0.3Co0.6Mn0.1)O2 and graphite are intimate, and the by-products are not found at the interphase. The discharge capacities of 80 mAh/g are obtained for 100 cycles at 150 °C when the charging voltage is restricted to 3.95 V. The results of cyclic voltammetry measurement indicate the reductive decomposition of the solid electrolyte at 2.3 and 1.6 V. These reduction currents are decreased with cycling, suggesting the passivation of the anode interphase. On the other hand, the oxidation current is observed above 3.6 V which is not terminated during voltage cycling. In the battery fabrication process, high-temperature sintering is not necessary, and the dense contacts with electrode materials can be made by hot pressing at 250 °C. Furthermore, the batteries are constructed in a dry room where the dew point is maintained at − 40 °C. The present results suggest the potential of the LiOH-Li2SO4 as the solid electrolyte for all-solid-state lithium batteries.







Similar content being viewed by others
References
Pan H, Cheng Z, He P, Zhou H (2020) A review of solid-state lithium–sulfur battery: ion transport and polysulfide chemistry. Energy Fuels 34:11942–11961. https://doi.org/10.1021/acs.energyfuels.0c02647
Hikima K, Suzuki K, Taminato S, Hirayama M, Yasuno S, Kanno R (2019) Thin film all-solid-state battery using Li2MnO3 epitaxial film electrode. Chem Lett 48:192–195. https://doi.org/10.1246/cl.180773
Kobayashi Y, Takeuchi T, Tabuchi M, Kageyama H (1999) Densification of LiTi2(PO4)3-based solid electrolytes by spark-plasma-sintering. J Power Sources 81–82:853–858
Sakuda A, Hayashi A, Tatsumisago M (2013) Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci Rep 3:2261. https://doi.org/10.1038/srep02261
Unemoto A et al (2014) Development of bulk-type all-solid-state lithium-sulfur battery using LiBH4 electrolyte. Appl Phys Lett 105:083901. https://doi.org/10.1063/1.4893666
Unemoto A, Yoshida K, Ikeshoji T, Orimo S-I (2016) Bulk-type all-solid-state lithium batteries using complex hydrides containing cluster-anions. Mater Trans 57:1639–1644. https://doi.org/10.2320/matertrans.MAW201601
Yoshikawa K, Yamamoto T, Sugumar MK, Motoyama M, Iriyama Y (2021) Room temperature operation and high cycle stability of an all-solid-state lithium battery fabricated by cold pressing using soft Li2OHBr solid electrolyte. Energy Fuels 35:12581–12587. https://doi.org/10.1021/acs.energyfuels.1c01190
Unemoto A et al (2015) Stable interface formation between TiS2 and LiBH4 in bulk-type all-solid-state lithium batteries. Chem Mater 27:5407–5416. https://doi.org/10.1021/acs.chemmater.5b02110
Sugumar MK, Yamamoto T, Motoyama M, Iriyama Y (2020) Room temperature synthesis of anti-perovskite structured Li2OHBr. Solid State Ionics 349:115298. https://doi.org/10.1016/j.ssi.2020.115298
Gao L, Zhao R, Han S, Li S, Zou R, Zhao Y (2021) Antiperovskite ionic conductor Layer for stabilizing the interface of NASICON solid electrolyte against li metal in all-solid-state batteries. Batteries Supercaps 4:1491–1498. https://doi.org/10.1002/batt.202100123
Tatsumisago M, Takano R, Tadanaga K, Hayashi A (2014) Preparation of Li3BO3–Li2SO4 glass–ceramic electrolytes for all-oxide lithium batteries. J Power Sources 270:603–607. https://doi.org/10.1016/j.jpowsour.2014.07.061
Nagao K, Nose M, Kato A, Sakuda A, Hayashi A, Tatsumisago M (2017) Preparation and characterization of glass solid electrolytes in the pseudoternary system Li3BO3-Li2SO4-Li2CO3. Solid State Ionics 308:68–76. https://doi.org/10.1016/j.ssi.2017.05.009
Biefeld RM, Johnson RT, J. (1979) The effects of Li2SO4 addition, moisture, and LiOH on the ionic conductivity of Li5AlO4. J Solid State Chem 29:393–399
Deshpande VK, Raghuwanshi FC, Singh K (1986) Electrical conductivity of the Li2SO4-LiOH system. Solid State Ionics 18&19:378–381
Singh K, Raghuwanshi FC (1988) Li2SO4_LiOH eutectic system, a promising solid electrolyte. Solid State Ionics 28–30:267–270
Dai P, Kong X, Yang H, Li J, Zeng J, Zhao J (2022) Single-crystal Ni-Rich layered LiNi0.9Mn0.1O2 enables superior performance of Co-free cathodes for lithium-ion batteries. ACS Sustainable Chemistry & Engineering 10:4381–4390. https://doi.org/10.1021/acssuschemeng.1c06704
Li S et al (2022) (2022) Thermal-healing of lattice defects for high-energy single-crystalline battery cathodes. Nat Commun 13:704. https://doi.org/10.1038/s41467-022-28325-5
Qian G et al (2022) Value-creating upcycling of retired electric vehicle battery cathodes. Cell Reports Physical Science 3:100741. https://doi.org/10.1016/j.xcrp.2022.100741
Takamori S, Doi T, Inaba M (2023) Aluminum doping effects on large LiNi0.8Co0.1Mn0.1O2 single crystal particles prepared in a molten LiOH-Li2SO4 flux. J Electrochem Soc 170:020532. https://doi.org/10.1149/1945-7111/acbc51
Strauss F et al (2020) Rational Design of Quasi-Zero-Strain NCM cathode materials for minimizing volume change effects in all-solid-state batteries. ACS Materials Letters 2:84–88. https://doi.org/10.1021/acsmaterialslett.9b00441
Tatsumisago M et al (2017) Electrical and mechanical properties of glass and glass-ceramic electrolytes in the system Li3BO3-Li2SO4. J Ceram Soc Jpn 125:433–437. https://doi.org/10.2109/jcersj2.17026
Hensley DA, Garofalini SH (1994) (1994) XPS investigation of lithium borate glass and the Li_LiBO2 interface. Appl Surf Sci 81:331–339
Dudenkov IV, Solntsev KA (2009) Theoretical prediction of the new high-density lithium boride LiB11 with polymorphism and pseudoplasticity. Russ J Inorg Chem 54:1261–1272. https://doi.org/10.1134/s0036023609080142
Ding X, Xin Y, Wang Y, Wang M, Song T, Gao H (2023) Artificial solid electrolyte interphase engineering toward dendrite-free lithium anodes. ACS Sustainable Chemistry & Engineering 11:6879–6889. https://doi.org/10.1021/acssuschemeng.2c06146
Zhu Y, He X, Mo Y (2015) Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl Mater Interfaces 7:23685–23693. https://doi.org/10.1021/acsami.5b07517
Guo L et al (2021) The electrolysis of anti-perovskite Li2OHCl for prelithiation of high-energy-density batteries. Angew Chem Int Ed Engl 60:13013–13020. https://doi.org/10.1002/anie.202102605
Posada-Pérez S, Rignanese G-M, Hautier G (2021) Influence of stacking on H+ intercalation in layered ACoO2 (A = Li, Na) cathode materials and implications for aqueous Li-Ion batteries: a first-principles investigation. Chem Mater 33:6942–6954. https://doi.org/10.1021/acs.chemmater.1c01887
Reddy IN et al (2020) A systematic study of annealing environment and Al dopant effect on NASICON-type LiZr2(PO4)3 solid electrolyte. Ionics 26:4287–4298. https://doi.org/10.1007/s11581-020-03622-5
Akkinepally B et al (2022) Dopant effect on Li+ ion transport in NASICON-type solid electrolyte: insights from molecular dynamics simulations and experiments. Ceram Int 48(9):12142–12151. https://doi.org/10.1016/j.ceramint.2022.01.075
Acknowledgements
This work was supported by the NGK Environment Innovation Laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miyazaki, R., Yagi, E., Ozaki, S. et al. Ionic conductivity of the LiOH-Li2SO4 system and fabrication of all-solid-state lithium batteries. J Solid State Electrochem 28, 103–111 (2024). https://doi.org/10.1007/s10008-023-05656-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05656-x