Abstract
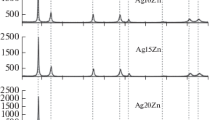
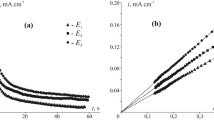
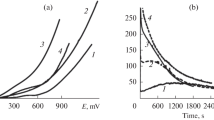
Silver(I) oxide was anodically formed in a deaerated 0.1 M KOH aqueous solution on Ag and Ag–Zn alloys (from 5 to 30 at.% of zinc) subjected to anodic selective dissolution in a deaerated 0.01 M HNO3 + 0.09 М KNO3 aqueous solution. Under these conditions, a layer is formed on the surface of the alloy enriched with silver and structural defects whose concentration is significantly higher than the equilibrium one. Scanning electron microscopy, impedancemetry, and photopotential measurements were used. It was determined that irrespective of the concentration of the non-equilibrium defects in the surface layer of the alloy, the n-type silver(I) oxide was formed with prevailing donor defects, spheroid morphology, and a relatively low chemical stability in an aqueous alkaline solution. The higher the concentration of zinc in the alloy and structural defects in its surface layer, the lower the diameter of silver(I) oxide crystallites. An increase in the flat band potential of silver(I) oxide from 0.428 to 0.454 V following an increase in the concentration of superequilibrium defects in the alloy’s surface layer from 17.1⋅10−4 to 65.3⋅10−4 at.% is only observed for the alloy with the concentration of zinc of 5 at.%. The concentration of donor defects in the silver(I) oxide generally increases following an increase in the concentration of zinc in the alloy. The dependence of concentration of donor defects in the silver(I) oxide on the concentration of superequilibrium defects in the alloys surface layer is non-monotonic. The rate constant of chemical dissolution of the silver(I) oxide decreases following both an increase in the concentration of zinc in the alloy and an increase in the concentration of defects in its surface layer.





Similar content being viewed by others
Notes
The experiments were performed at the VSU Centre for Collective Use of Scientific Equipment.
References
Rafieerad AR, Bushroa AR, Nasiri-Tabrizi B, Vadivelu J, Basirun WJ (2016) Silver/silver oxide nanorod arrays from physical vapor deposition and subsequent anodization processes. Surf Coat Technol 302:275–283
Sagadevan S (2016) Synthesis, structural, surface morphology, optical and electrical properties of silver oxide nanoparticles. Int J Nanoelectron Mater 9:37–49
Ferretti AM, Ponti A, Molteni G (2015) Silver(I) oxide nanoparticles as a catalyst in the azide–alkyne cycloaddition. Tetrahedron Lett 56:5727–5730
Li H-H, He Yi, Jin P-P, Cao Y, Fan M-H, Zou X, Li G-D (2016) Highly selective detection of trace hydrogen against CO and CH4 by Ag/Ag2O–SnO2 composite microstructures. Sens Actuators B 228:515–522
Shoeb M, Mobin M, Ahmad S, Naqvi AH (2021) Facile synthesis of polypyrrole coated graphene Gr/AgeAg2O/PPy nanocomposites for a rapid and selective response towards ammonia sensing at room temperature. Journal of Science: Adv Mater Devices 6:223–233
Rahman MM, Khan SB, Jamal A, Faisal M, Asiri AM (2012) Fabrication of highly sensitive acetone sensor based on sonochemically prepared as-grown Ag2O nanostructure. Chem Eng J 192:122–128
Rahman MM, Khan SB, Jamal A, Faisal M, Asiri AM (2012) Highly sensitive methanol chemical sensor based on undoped silver oxide nanoparticles prepared by a solution method. Microchim Acta 178:99–106
Quan H, Park S-U, Park J (2010) Electrochemical oxidation of glucose on silver nanoparticle-modified composite electrodes. Electrochim Acta 55:2232–2237
Deekshitha, Vidya Shetty K (2021) Solar light active biogenic titanium dioxide embedded silver oxide (AgO/ Ag2O@TiO2) nanocomposite structures for dye degradation by photocatalysis. Mater Sci Semicond Process 132:105923
Saraswathi B, Patil VS, Halse SV, Kalasad MN (2022) Optical and structural studies of PVP capped silver oxide nanoparticles. Materials Today: Proceedings 67:290–294
Fang C, Ellis AV, Voelker NH (2012) Electrochemical synthesis of silver oxide nanowires, microplatelets and application as SERS substrate precursors. Electrochim Acta 59:346–353
Singh PK, Bishwakarma H, Shubham, Das AK (2017) Study of annealing effects on Ag2O nanoparticles generated by electrochemical spark process. J Electron Mater 46:5715–5727
Gao X, Zhao M, Zhang Z, Chen C, Jiaomin M, Lu J (2011) Effects of hydrogen annealing on the microstructure and optical properties of single-phased Ag2O film deposited using direct-current reactive magnetron sputtering. Thin Solid Films 519:6620–6623
Alhasan SFH, Bader BA, Salim ET (2021) Surface morphology and roughness of silver oxide prepared employing pulsed laser at optimum laser fluence. Materials Today: Proceedings 42:2845–2848
Pierson JF, Rousselot C (2005) Stability of reactively sputtered silver oxide films. Surf Coat Technol 200:276–279
Büchel D, Tominaga J, Fukaya T, Atoda N (2001) Spectroscopic investigation of AgOx films for super resolution near field structure application. J Magn Soc Jpn 25:240–243
Fujimaki M, Iwanabe Y, Awazu K, Tominaga J (2006) Molecular detection in a micro channel using silver-oxide thin film. Microelectron Eng 83:1626–1629
Büchel D, Mihalcea C, Fukaya T, Atoda N, Tominaga J (2001) Sputtered silver oxide layers for surface-enhanced Raman spectroscopy. Appl Phys Lett 79:620–622
Peyser LA, Lee T, Dickson RM (2002) Mechanism of Agn nanocluster photoproduction from silver oxide films. J Phys Chem B 106:7725–7728
Trabanelli G, Zucchi F, Brunoro G, Bolognesi GP (1972) Photopotential measurements in the study of surface layers in metal corrosion, inhibition and passivation phenomena. Thin Solid Films 13:131–142
Liu X-Y, Zhou G-D, Yang M-Z, Cai S-M, Loo BH (1993) A simple method for monitoring the inhibition of copper corrosion based on photopotential measurements. J Electroanal Chem 361:265–267
Sutter EMM, Millet B, Fiaud C, Lincot D (1995) Some new photoelectrochemical insights in the corrosion-passivation processes of copper in aqueous chloride solution under open-circuit conditions. J Electroanal Chem 386:101–119
Jayram ND, Aishwarya D, Sonia S, Mangalaraj D, Kumar PS, Rao GM (2016) Analysis on superhydrophobic silver decorated copper oxide nanostructured thin films for SERS studies. J Colloid Interface Sci 477:209–219
Vvedenskii A, Grushevskaya S, Kudryashov D, Ganzha S (2010) The influence of the conditions of the anodic formation and the thickness of silver(I) oxide nanofilm on its semiconductor properties J Solid State Electrochem 14:1401–1413
Kudryashov DA, Grushevskaya SN, Vvedensky AV (2008) Division of partial rates of copper ionization, anodic oxide formation and chemical dissolution of Cu (I) oxide by chronoammetry of RRDE. Metal Protection 44:301–329
Ghahramanzadeh Asl H, Sert Y, Küçükömeroğlu T, Bayrak Ö (2023) The comparison of wear performances of CP-Ti, Ti6Al4V, Ti45Nb alloys oxidized by anodic oxidation under ambient air and vacuum conditions. Mater Today Commun 34:105466
Maciej A, Wadas A, Sowa M, Socha R, Kubiczek M, Simka W (2021) Colourful thin passive films on a Zn-Co alloy formed by anodic oxidation. Electrochim Acta 373:137922
Moon S, Nam Y (2012) Anodic oxidation of Mg–Sn alloys in alkaline solutions. Corros Sci 65:494–501
Masahashi N, Mizukoshi Y, Inoue H, Ohmura K, Moroishi T (2016) Photo-induced properties of anodic oxide on Ti–Pd alloy prepared in acetic acid electrolyte J Alloys Compd 669:91–100
Feliu S Jr, Bartolomé MJ, González JA, López V, Feliu S (2008) Passivating oxide film and growing characteristics of anodic coatings on aluminium alloys. Appl Surf Sci 254:2755–2762
Kamkin AN, Zhou G-D, Davydov AD (1999) Photoelectrochemical characteristics of oxide layers on copper-nickel alloys. J Solid State Electrochem 3:457–463
Bocharnikova MY, Murtazin MM, Grushevskaya SN, Kozaderov OA, Vvedensky AV (2022) Anodic formation and properties of nanoscale oxide layers on silver-zinc alloys with different concentrations of non-equilibrium vacancies. J Solid State Electrochem 26:1637–1644
Pickering HW, Wagner C (1967) Electrolytic dissolution of binary alloys containing a noble metal. J Electrochem Soc 114:698–706
Hoshi Y, Ozawa R, Tada E, Nishikata A, Tsuru T (2012) Selective dissolution of binary Pt–Co alloys of different compositions in sulphuric acid solution. Corros Sci 65:512–519
Nikitina EV, Karfidov EA, Kazakovtseva NA (2020) Anodic selective dissolution of copper alloys in chloride and carbonate melts. J Alloys Compd 845:156238
Kozaderov OA, Vvedensky AV (2014) Mass transfer and phase formation during anodic selective dissolution of homogeneous alloys: a monograph. Nauchnaya kniga, Voronezh
Spassov T, Lyubenova L, Liu Y, Bliznakov S, Spassova M, Dimitrov N (2009) Mechanochemical synthesis, thermal stability and selective electrochemical dissolution of Cu–Ag solid solutions. J Alloys Compd 478:232–236
Gurevich YY, Pleskov YV (1983) Photoelectrochemistry of semiconductors. Nauka, Moscow
Murtazin MM, Nesterova MY, Grushevskaya SN, Vvedensky AV (2019) Silver(I) oxide on silver–zinc alloys: anodic formation and properties. Russ J Electrochem 55:680–689
Grdeń M (2017) Impedance study on the capacitance of silver electrode oxidised in alkaline electrolyte. J Solid State Electrochem 21:3333–3344
Hecht D, Borthen P, Strehblow H-H (1995) In situ examination of anodic silver oxide films by EXAFS in the reflection mode. J Electroanal Chem 381:113–121
Kudryashov DA, Grushevskaya SN, Ganzha SV, Vvedensky AV (2009) Effect of the crystal face orientation and alloying with gold on the properties of thin anodic films of Ag(I) oxide. Part I. Photocurrent. Prot Met Phys Chem Surf and 45:451–460
Encyclopedia of electrochemistry. V.6: semiconductor electrodes and photoelectrochemistry (2002) Bard AJ, Stratmann M, Licht S (Eds) Wiley-VCH, Weinheim
New handbook for a chemist and technologist (2006) Moskvina AV (Ed) Professional, St. Petersburg
Singh N, Choudhary S, Upadhyay S, Satsangi VR, Dass S, Shrivastav R (2014) Nanocrystalline Zn1-xAgxOy thin films evolved through electrodeposition for photoelectrochemical splitting of water J Solid State Electrochem 18:523–533
Acknowledgements
The study was supported by the Ministry of Science and Higher Education of the Russian Federation under Agreement N 075-15-2021-1351 in part of functional materials properties analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bocharnikova, M.Y., Grushevskaya, S.N., Kozaderov, O.A. et al. Morphology, semiconductor properties, and chemical stability of AG(I) oxide anodically formed on silver and silver alloys. J Solid State Electrochem 28, 243–253 (2024). https://doi.org/10.1007/s10008-023-05654-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05654-z