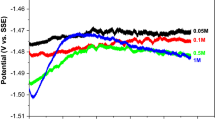
Abstract
Effects of MnO2 electrodeposition on α, β, γ, and δ-MnO2 polymorphs from aqueous zinc sulfate solution with manganese sulfate additive (zinc-ion battery (ZIB) electrolyte) have been examined by cyclic voltammetry, electrochemical impedance spectroscopy, X-ray diffraction, and scanning electron microscopy. Even three cycles of anodic charge and cathodic discharge in the typical potential range used in zinc-ion battery research are sufficient for entire electrode surface coverage by essentially X-ray amorphous deposit with a minor contribution of γ-MnO2. The fast MnO2 deposition proceeds via Mn2+ anodic oxidation upon charge at potentials above 1.8 V (vs. Zn2+/Zn). As a consequence of the fast electrodeposition, the choice of MnO2 polymorph for the positive electrode in aqueous ZIB with Mn(II) additive in the electrolyte turns to be even less critical than in ZIB without Mn(II) additive. Though using a narrower potential range in the battery charging may help to mitigate the MnO2 electrodeposition, the cost of the mitigation would be a reduction of an enhanced capacity of Mn(II)-containing ZIB, as the latter is essentially due to MnO2 anodic deposition.
Graphical abstract






Similar content being viewed by others
References
Xu C, Du H, Li B, Kang F, Zeng Y (2009) Reversible insertion properties of zinc ion into manganese dioxide and its application for energy storage. Electrochem Solid-State Lett 12(4):A61. https://doi.org/10.1149/1.3065967
Xu C, Li B, Du H, Kang F (2012) Energetic zinc ion chemistry: the rechargeable zinc ion battery. Angew Chem Int Ed 51(4):933–935. https://doi.org/10.1002/anie.201106307
Ma N, Wu P, Wu Y, Jiang D, Lei G (2019) Progress and perspective of aqueous zinc-ion battery. Funct Mater Lett 12(05):1930003. https://doi.org/10.1142/S1793604719300032
Konarov A, Voronina N, Jo JH, Bakenov Z, Sun YK, Myung ST (2018) Present and future perspective on electrode materials for rechargeable zinc-ion batteries. ACS Energy Lett 3(10):2620–2640. https://doi.org/10.1021/acsenergylett.8b01552
Blanc LE, Kundu D, Nazar LF (2020) Scientific challenges for the implementation of Zn-ion batteries. Joule 4(4):771–799. https://doi.org/10.1016/j.joule.2020.03.002
Parsons R (1974) Electrochemical nomenclature. Pure Appl Chem 37(4):499–516. https://doi.org/10.1351/pac197437040499
Guo X, Zhou J, Bai C, Li X, Fang G, Liang S (2020) Zn/MnO2 battery chemistry with dissolution-deposition mechanism. Mater Today Energy 16:100396. https://doi.org/10.1016/j.mtener.2020.100396
Stoševski I, Bonakdarpour A, Fang B, Lo P, Wilkinson DP (2021) Formation of MnxZny(OH)zSO4⋅5H2O – not intercalation of Zn – is the basis of the neutral MnO2/Zn battery first discharge reaction. Electrochim Acta 390:138852. https://doi.org/10.1016/j.electacta.2021.138852
Pan H, Shao Y, Yan P, Cheng Y, Han KS, Nie Z, Wang C, Yang J, Li X, Bhattacharya P, Mueller KT, Liu J (2016) Reversible aqueous zinc/manganese oxide energy storage from conversion reactions. Nat Energy 1:1–7. https://doi.org/10.1038/nenergy.2016.39
Sun W, Wang F, Hou S, Yang C, Fan X, Ma Z, Gao T, Han F, Hu R, Zhu M, Wang C (2017) Zn/MnO2 battery chemistry with H+ and Zn2+ coinsertion. J Am Chem Soc 139(29):9775–9778. https://doi.org/10.1021/jacs.7b04471
Islam S, Alfaruqi MH, Mathew V, Song J, Kim S, Kim S, Jo J, Baboo JP, Pham DT, Putro DY, Sun YK, Kim J (2017) Facile synthesis and the exploration of the zinc storage mechanism of β-MnO2 nanorods with exposed (101) planes as a novel cathode material for high performance eco-friendly zinc-ion batteries. J Mater Chem A 5(44):23299–23309. https://doi.org/10.1039/C7TA07170A
Qiu N, Chen H, Yang Z, Sun S, Wang Y (2018) Low-cost birnessite as a promising cathode for high-performance aqueous rechargeable batteries. Electrochim Acta 272:154–160. https://doi.org/10.1016/j.electacta.2018.04.012
Li Y, Wang S, Salvador JR, Wu J, Liu B, Yang W, Yang J, Zhang W, Yang J (2018) Reaction mechanisms for long life and ultra-high power rechargeable Zn ion batteries. Meet Abstr MA2018–01:206. https://doi.org/10.1149/MA2018-01/2/206
Alfaruqi MH, Gim J, Kim S, Song J, Pham DT, Jo J, Xiu Z, Mathew V, Kim J (2015) Layered δ-MnO2 nanoflake cathode with high zinc-storage capacities for eco-friendly battery applications. Electrochem Commun 60:121–125. https://doi.org/10.1016/j.elecom.2015.08.019
Alfaruqi MH, Mathew V, Gim J, Kim S, Song J, Baboo JP, Choi SH, Kim J (2015) Electrochemically induced structural transformation in a γ-MnO2 cathode of a high capacity zinc-ion battery system. Chem Mater 27(10):3609–3620. https://doi.org/10.1021/cm504717p
Zhao S, Han B, Zhang D, Huang Q, Xiao L, Chen L, Ivey DG, Deng Y, Wei W (2018) Unravelling the reaction chemistry and degradation mechanism in aqueous Zn/MnO2 rechargeable batteries. J Mater Chem A 6(14):5733–5739. https://doi.org/10.1039/C8TA01031E
Yuan C, Zhang Y, Pan Y, Liu X, Wang G, Cao D (2014) Investigation of the intercalation of polyvalent cations (Mg2+, Zn2+) into λ-MnO2 for rechargeable aqueous battery. Electrochim Acta 116:404–412. https://doi.org/10.1016/j.electacta.2013.11.090
Xu D, Li B, Wei C, He YB, Du H, Chu X, Qin X, Yang QH, Kang F (2014) Preparation and characterization of MnO2/acid-treated CNT nano composites for energy storage with zinc ions. Electrochim Acta 133:254–261. https://doi.org/10.1016/j.electacta.2014.04.001
Gao X, Wu H, Li W, Tian Y, Zhang Y, Wu H, Yang L, Zou G, Hou H, Ji X (2020) H+-insertion boosted α-MnO2 for an aqueous Zn-ion battery. Small 16(5):1905842. https://doi.org/10.1002/smll.201905842
Chamoun M, Brant WR, Tai CW, Karlsson G, Noréus D (2018) Rechargeability of aqueous sulfate Zn/MnO2 batteries enhanced by accessible Mn2+ ions. Energy Storage Mater 15:351–360. https://doi.org/10.1016/j.ensm.2018.06.019
Huang C, Wu C, Zhang Z, Xie Y, Li Y, Yang C, Wang H (2021) Crystalline and amorphous MnO2 cathodes with open framework enable high-performance aqueous zinc-ion batteries. Front Mater Sci 15(2):202–215. https://doi.org/10.1007/s11706-021-0551-y
Tang Z, Chen W, Lyu Z, Chen Q (2022) Size-dependent reaction mechanism of λ-MnO2 Particles as cathodes in aqueous zinc-ion batteries. Energy Mater Adv 2022:9765710. https://doi.org/10.34133/2022/9765710
Wu B, Zhang G, Yan M, Xiong T, He P, He L, Xu X, Mai L (2018) Graphene scroll-coated α-MnO2 nanowires as high-performance cathode materials for aqueous Zn-ion battery. Small 14(13):1703850. https://doi.org/10.1002/smll.201703850
Huang J, Wang Z, Hou M, Dong X, Liu Y, Wang Y, Xia Y (2018) Polyaniline-intercalated manganese dioxide nanolayers as a high-performance cathode material for an aqueous zinc-ion battery. Nat Commun 9:2906. https://doi.org/10.1038/s41467-018-04949-4
Cai X, Li H, Li J, Yan H, Liu Y, Yu H, Yan L, Zhang L, Shu J (2021) Hydrothermal synthesis of β-MnO2 nanorods for highly efficient zinc-ion storage. Ionics 27:3943–3950. https://doi.org/10.1007/s11581-021-04188-6
Siamionau U, Aniskevich Y, Mazanik A, Kokits O, Ragoisha G, Jo JH, Myung ST, Streltsov E (2022) Rechargeable zinc-ion batteries with manganese dioxide cathode: how critical is choice of manganese dioxide polymorphs in aqueous solutions? J Power Sources 523:231023. https://doi.org/10.1016/j.jpowsour.2022.231023
Wang X, Li Y (2003) Synthesis and formation mechanism of manganese dioxide nanowires/nanorods. Chem Eur J 9(1):300–306. https://doi.org/10.1002/chem.200390024
Duan X, Yang J, Gao H, Ma J, Jiao L, Zheng W (2012) Controllable hydrothermal synthesis of manganese dioxide nanostructures: shape evolution, growth mechanism and electrochemical properties. CrystEngComm 14(12):4196–4204. https://doi.org/10.1039/C2CE06587H
Bondarenko AS, Ragoisha GA (2013) EIS spectrum analyser. http://www.abc.chemistry.bsu.by/vi/analyser/. Accessed 15 Jan 2023
Haynes WM (ed) (2016) CRC handbook of chemistry and physics. CRC Press. https://doi.org/10.1201/9781315380476
Kitchaev DA, Dacek ST, Sun W, Ceder G (2017) Thermodynamics of phase selection in MnO2 framework structures through alkali intercalation and hydration. J Am Chem Soc 139(7):2672–2681. https://doi.org/10.1021/jacs.6b11301
Turner S, Buseck PR (1979) Manganese oxide tunnel structures and their intergrowths. Science 203(4379):456–458. https://doi.org/10.1126/science.203.4379.456
Sokolsky GV, Boldyrev YI, Ivanova ND, Ivanov SV, Kolbasov GY, Lazzara G, Zudina LV, Gayuk NV, Chivikov SV (2020) Effects of electrolyte doping on electrodeposited nanostructured manganese oxide and chromium oxide. Surf Coat Technol 400:126211. https://doi.org/10.1016/j.surfcoat.2020.126211
Ragoisha GA, Auchynnikava TA, Streltsov EA, Rabchynski SM (2014) Electrochemical impedance of platinum in concentrated chloride solutions under potentiodynamic anodic polarization: effect of alkali metal cations. Electrochim Acta 122:218–223. https://doi.org/10.1016/j.electacta.2013.09.139
Funding
This research has received funding from the Himreagent program 2021–2025 (nos. 20210562 and 20211465).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Siamionau, U.V., Aniskevich, Y.M., Ragoisha, G.A. et al. MnO2 electrodeposition at the positive electrode of zinc-ion aqueous battery containing Zn2+ and Mn2+ cations. J Solid State Electrochem 27, 1911–1918 (2023). https://doi.org/10.1007/s10008-023-05467-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05467-0