Abstract
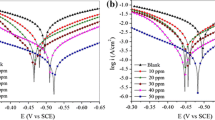
In this work, six groups of non-toxic compounds were tested as inhibitors for mild steel in 1 M HCl solution: glycine (Gly), glutamic acid (Glu), cysteine (Cys), a mixture of three amino acids glycine, glutamic acid, and cysteine (Gly + Glu + Cys); a mixture of dipeptide composed of glycine and glutamic acid with amino acid cysteine (Gly-Glu + Cys); and a tripeptide composed of glycine, cysteine and glutamic acid which is called glutathione (Glt). The inhibition performances of inhibitor systems for steel corrosion were investigated by electrochemical tests (polarization measurements and electrochemical impedance spectroscopy) and surface analyses (atomic force microscopy-AFM, optical microscope, and photoelectron spectroscopy-XPS). Experimental results showed that all six groups of inhibitors affect the reduction of steel corrosion rate, with Glt having the highest efficiency during 4-h immersion (97.3%). Atomic force microscopy and optical microscope showed that the inhibitors are able to protect the metal surface and reduce the extent of corrosion. The existence of the Glt inhibitory film on the steel surface was confirmed by the XPS method. DFT calculations provided useful insights into adsorption of the corrosion inhibitors.










Similar content being viewed by others
References
Dehghani A, Bahlakeh G, Ramezanzadeh B, Ramezanzadeh M (2019) Potential of borage flower aqueous extract as an environmentally sustainable corrosion inhibitor for acid corrosion of mild steel. Electrochemical and theoretical studies. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.01.008
Fernandes CM, da Ferreira Fagundes T, S, Escarpini dos Santos N, Rocha TS de M, Garrett R, Borges RM, Muricy G, Valverde AL, Ponzio EA (2019) Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim Acta. https://doi.org/10.1016/j.electacta.2019.04.148
Aslam R, Mobin M, Zehra S, Aslam J (2022) A comprehensive review of corrosion inhibitors employed to mitigate stainless steel corrosion in different environments. J Mol Liq. https://doi.org/10.1016/j.molliq.2022.119992
Oubaaqa M, Ouakki M, Rbaa M, Abousalem AS, Maatallah M, Benhiba F, Jarid A, Ebn Touhami M, Zarrouk A (2021) Insight into the corrosion inhibition of new amino-acids as efficient inhibitors for mild steel in HCl solution: experimental studies and theoretical calculations. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116520
Umoren SA, Solomon MM, Obot IB, Suleiman RK (2019) A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2019.03.057
Ahamad I, Prasad R, Quraishi MA (2010) Experimental and theoretical investigations of adsorption of fexofenadine at mild steel/hydrochloric acid interface as corrosion inhibitor. J Solid State Electrochem. https://doi.org/10.1007/s10008-010-1041-9
Goni LKMO, Mazumder MAJ (2019) Green corrosion inhibitors. INTECH. https://doi.org/10.5772/intechopen.81376
Tan B, Xiang B, Zhang S, Qiang Y, Xu L, Chen S, He J (2021) Papaya leaves extract as a novel eco-friendly corrosion inhibitor for Cu in H2SO4 medium. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2020.08.093
Alibakhshi E, Ramezanzadeh M, Bahlakeh G, Ramezanzadeh B, Mahdavian M, Motamedi M (2018) Glycyrrhiza glabra leaves extract as a green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution: experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.01.144
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Saraca ashoka extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Mol Liq. https://doi.org/10.1016/j.molliq.2018.02.104
Qiang Y, Zhang S, Tan B, Chen S (2018) Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros Sci. https://doi.org/10.1016/j.corsci.2018.01.008
Marzorati S, Verotta L, Trasatti SP (2019) Green corrosion inhibitors from natural sources and biomass wastes. Molecules. https://doi.org/10.3390/molecules24010048
Ituen E, Mkpenie V, Ekemini E (2019) Corrosion inhibition of X80 steel in simulated acid wash solution using glutathione and its blends: experimental and theoretical studies. Colloids Surfaces A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2019.123597
Prasad D, Singh R, Kaya S, Ibrahimi B (2022) Natural corrosion inhibitor of renewable eco-waste for SS-410 in sulfuric acid medium: adsorption, electrochemical, and computational studies. J Mol Liq. https://doi.org/10.1016/j.molliq.2022.118671
Wang Q, Tan B, Bao H, Xie Y, Mou Y, Li P, Chen D, Shi Y, Li X, Yang W (2019) Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry. https://doi.org/10.1016/j.bioelechem.2019.03.001
Shehata OS, Korshed LA, Attia A (2018) Green corrosion inhibitors, past, present, and future. Corros Inhib Princ Recent Appl. https://doi.org/10.5772/intechopen.72753
Gupta DK (2013) Green inhibitors for prevention of metal and alloys corrosion : an overview 3:16–24
Kesavan D, Gopiraman M, Sulochana N (2012) Green inhibitors for corrosion of metals : a review. Chem Sci Rev Lett 1:1–8
El Ibrahimi B, Baddouh A, Oukhrib R, El Issami S, Hafidi Z, Bazzi L (2021) Electrochemical and in silico investigations into the corrosion inhibition of cyclic amino acids on tin metal in the saline environment. Surfaces and Interfaces. https://doi.org/10.1016/j.surfin.2021.100966
Kasprzhitskii A, Lazorenko G (2021) Corrosion inhibition properties of small peptides: DFT and Monte Carlo simulation studies. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.115782
Zhang DQ, Xie B, Gao LX, Cai QR, Joo HG, Lee KY (2011) Intramolecular synergistic effect of glutamic acid, cysteine and glycine against copper corrosion in hydrochloric acid solution. Thin Solid Films. https://doi.org/10.1016/j.tsf.2011.07.009
Khaled KF (2009) Monte Carlo simulations of corrosion inhibition of mild steel in 0.5 M sulphuric acid by some green corrosion inhibitors. J Solid State Electrochem. https://doi.org/10.1007/s10008-009-0845-y
Unnimaya SP, Kumari P, Kagatikar S (2022) Glutathione as green corrosion inhibitor for 6061Al-SiC(p) composite in HCl medium: electrochemical and theoretical investigation. J Solid State Electrochem. https://doi.org/10.1007/s10008-022-05315-7
Chauhan DS, Quraishi MA, Srivastava V, Haque J, El ibrahimi B (2021) Virgin and chemically functionalized amino acids as green corrosion inhibitors: influence of molecular structure through experimental and in silico studies. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.129259
Monticelli C (2018) Corrosion inhibitors, Encyclopedia of Interfacial Chemistry, Surf Sci Electrochem 164–171
Kumar D, Jain N, Jain V, Rai B (2020) Amino acids as copper corrosion inhibitors: a density functional theory approach. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2020.145905
Xu XT, Xu HW, Li W, Wang Y, Zhang Y (2022) A combined quantum chemical, molcular dynamics and Monto Carlo study of three amino acids as corrosion inhibitors for aluminum in NaCl solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.117010
Yeganeh M, Rezvani H, Laribaghal SM (2021) Electrochemical behavior of additively manufactured 316L stainless steel in H2SO4 solution containing methionine as an amino acid. Colloids Surfaces A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2021.127120
El Ibrahimi B, Jmiai A, El Mouaden K, Oukhrib R, Soumoue A, El Issami S, Bazzi L (2020) Theoretical evaluation of some α-amino acids for corrosion inhibition of copper in acidic medium: DFT calculations. J King Saud Univ - Sci, Monte Carlo simulations and QSPR studies. https://doi.org/10.1016/j.jksus.2018.04.004
Ituen E, Akaranta O, James A (2016) Green anticorrosive oilfield chemicals from 5-hydroxytryptophan and synergistic additives for X80 steel surface protection in acidic well treatment fluids. J Mol Liq. https://doi.org/10.1016/j.molliq.2016.10.024
Ituen EB, Akaranta O, Umoren SA (2017) N-acetyl cysteine based corrosion inhibitor formulations for steel protection in 15% HCl solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2017.09.040
Abdallah M, Soliman KA, Alfattani R, Al-Gorair AS, Fawzy A, Ibrahim MAA (2022) Insight of corrosion mitigation performance of SABIC iron in 0.5 M HCl solution by tryptophan and histidine: experimental and computational approaches. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2022.02.007
Qian HC, Chang WW, Liu WL, Cui TY, Li Z, Guo DW, Kwok CT, Tam LM, Zhang DW (2022) Investigation of microbiologically influenced corrosion inhibition of 304 stainless steel by D-cysteine in the presence of Pseudomonas aeruginosa. Bioelectrochemistry. https://doi.org/10.1016/j.bioelechem.2021.107953
Zhang Y, Zhang S, Tan B, Guo L, Li H (2021) Solvothermal synthesis of functionalized carbon dots from amino acid as an eco-friendly corrosion inhibitor for copper in sulfuric acid solution. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2021.07.034
Xu XT, Xu HW, Cui YF, Li W, Wang Y, Zhang XY (2022) Molecular dynamics study of three amino acids as corrosion inhibitor for copper in hydrochloric acid solution. J Mol Model. https://doi.org/10.1007/s00894-022-05038-6
Nagalaxmi, Shetty P, Kumari PP (2020) Inhibitive action of glutathione reduced on the deterioration of AA6061 in 0.5M HCl. Tribol Ind. https://doi.org/10.24874/ti.785.10.19.02
Izadi M, Rad AR, Shahrabi T, Mohammadi I (2020) The combined action of L-cysteine and L-histidine as a significant eco-friendly protective system to enhance the corrosion protection performance of AA2024-T3 alloy in 0.1 M NaCl solution: electrochemical and surface studies. Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2020.122997
Zhang QH, Jiang ZN, Li YY, Wang X, Xiong W, Liu HF, Zhang GA (2022) In-depth insight into the inhibition mechanism of the modified and combined amino acids corrosion inhibitors: “intramolecular synergism” vs. “intermolecular synergism”. Chem Eng J. https://doi.org/10.1016/j.cej.2022.135439
Zhang QH, Li YY, Lei Y, Wang X, Liu HF, Zhang GA (2022) Comparison of the synergistic inhibition mechanism of two eco-friendly amino acids combined corrosion inhibitors for carbon steel pipelines in oil and gas production. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2022.152559
Zhao R, Yu Q, Niu L (2022) Corrosion inhibition of amino acids for 316L stainless steel and synergistic effect of I− ions: experimental and theoretical studies. Mater Corros. https://doi.org/10.1002/maco.202112511
Fawzi Nassar M, Zedan Taban T, Fadhel Obaid R, Hatem Shadhar M, Abdulkareem Almashhadani H, Kadhim MM, Liu P (2022) Study to amino acid-based inhibitors as an effective anti-corrosion material. J Mol Liq. https://doi.org/10.1016/j.molliq.2022.119449
Jassim GS, Al-Dhalimy AMB, Noori AS, Shadhar MH, Kadhim MM, Almashhadani HA, Rheima AM, Liu P (2022) Study the application of new type green corrosion inhibitors for iron metal. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2022.109650
Kasprzhitskii A, Lazorenko G, Nazdracheva T, Kukharskii A, Yavna V, Kochur A (2021) Theoretical evaluation of the corrosion inhibition performance of aliphatic dipeptides. New J Chem. https://doi.org/10.1039/d0nj05281g
Pracht P, Bohle F, Grimme S (2020) Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys Chem Chem Phys. https://doi.org/10.1039/c9cp06869d
Grimme S, Bannwarth C, Shushkov P (2017) A robust and accurate tight-binding quantum chemical method for structures, vibrational frequencies, and noncovalent interactions of large molecular systems parametrized for all spd-block elements (Z = 1–86). J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.7b00118
Ehlert S, Stahn M, Spicher S, Grimme S (2021) Robust and efficient implicit solvation model for fast semiempirical methods. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.1c00471
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.77.3865
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys doi 10(1063/1):3382344
Camacho RL, Montiel E, Jayanthi N, Pandiyan T, Cruz J (2010) DFT studies of α-diimines adsorption over Fen surface (n = 1, 4, 9 and 14) as a model for metal surface coating. Chem Phys Lett. https://doi.org/10.1016/j.cplett.2009.12.016
Weigend F, Häser M, Patzelt H, Ahlrichs R (1998) RI-MP2: optimized auxiliary basis sets and demonstration of efficiency. Chem Phys Lett. https://doi.org/10.1016/S0009-2614(98)00862-8
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta. https://doi.org/10.1007/BF00549096
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys. https://doi.org/10.1080/00268977000101561
Simon S, Duran M, Dannenberg JJ (1996) How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers? J Chem Phys. https://doi.org/10.1063/1.472902
Gaussian 09, Revision D.01, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr J E, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox J E, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin A J, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, and Fox DJ Gaussian, Inc., Wallingford CT, 2013.
Satpati S, Suhasaria A, Ghosal S, Saha A, Dey S, Sukul D (2021) Amino acid and cinnamaldehyde conjugated Schiff bases as proficient corrosion inhibitors for mild steel in 1 M HCl at higher temperature and prolonged exposure: detailed electrochemical, adsorption and theoretical study. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.115077
Ralkhal S, Shahrabi T, Ramezanzadeh B, Bahlakeh G (2019) A combined electrochemical, molecular dynamics, quantum mechanics and XPS analysis of the mild steel surface protected by a complex film composed of neodymium (III) and benzimidazole. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2018.09.064
Arrousse N, Salim R, Bousraf FZ, Ech-chihbi E, Hammouti B, Abdellaoui A, El Hajjaji F, Taleb M (2022) Experimental and theoretical study of xanthene derivatives as corrosion inhibitor for mild steel in hydrochloric acid solution. J Appl Electrochem. https://doi.org/10.1007/s10800-022-01705-x
Aslam R, Mobin M, Huda OIB, Alamri AH (2020) Ionic liquids derived from α-amino acid ester salts as potent green corrosion inhibitors for mild steel in 1M HCl. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.113982
Peng Y, Hughes AE, Deacon GB, Junk PC, Hinton BRW, Forsyth M, Mardel JI, Somers AE (2018) A study of rare-earth 3-(4-methylbenzoyl)-propanoate compounds as corrosion inhibitors for AS1020 mild steel in NaCl solutions. Corros Sci. https://doi.org/10.1016/j.corsci.2018.09.022
Dehghani A, Poshtiban F, Bahlakeh G, Ramezanzadeh B (2020) Fabrication of metal-organic based complex film based on three-valent samarium ions-[bis (phosphonomethyl) amino] methylphosphonic acid (ATMP) for effective corrosion inhibition of mild steel in simulated seawater. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2019.117812
Sedik A, Lerari D, Salci A, Athmani S, Bachari K, Gecibesler H, Solmaz R (2020) Dardagan Fruit extract as eco-friendly corrosion inhibitor for mild steel in 1 M HCl: electrochemical and surface morphological studies. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2019.12.006
Saxena A, Prasad D, Haldhar R, Singh G, Kumar A (2018) Use of Sida cordifolia extract as green corrosion inhibitor for mild steel in 0.5 M H2SO4. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2017.12.064
Zhang M, Guo L, Zhu M, Wang K, Zhang R, He Z, Lin Y, Leng S, Chikaodili Anadebe V, Zheng X (2021) Akebia trifoliate koiaz peels extract as environmentally benign corrosion inhibitor for mild steel in HCl solutions: integrated experimental and theoretical investigations. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2021.06.009
Tang M, Li X, Deng S, Lei R (2021) Synergistic inhibition effect of Mikania micrantha extract with KI on steel corrosion in H2SO4 solution. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.117926
Zhang X, Li W, Zuo X, Tan B, Xu C, Zhang S (2021) Investigating the inhibitive effect of Davidia involucrata leaf extract as a biological eco-friendly inhibitor for copper in acidic medium. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.115214
Li H, Zhang S, Qiang Y (2021) Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: electrochemical and theoretical explorations. J Mol Liq. https://doi.org/10.1016/j.molliq.2020.114450
Wu G, Lu C, Wu X, Zhang S, He F, Ling L (2004) X-ray photoelectron spectroscopy investigation into thermal degradation and stabilization of polyacrylonitrile fibers. J Appl Polym Sci. https://doi.org/10.1002/app.21081
Chiang CL, Yang JM (2017) Flame retardance and thermal stability of polymer/graphene nanosheet oxide composites. Elsevier Ltd. https://doi.org/10.1016/b978-0-08-100136-3.00011-x
Xu C, Li W, Tan B, Zuo X, Zhang S (2022) Adsorption of Gardenia jasminoides fruits extract on the interface of Cu/H2SO4 to inhibit Cu corrosion: experimental and theoretical studies. J Mol Liq. https://doi.org/10.1016/j.molliq.2021.116996
Liu Q, Song Z, Han H, Donkor S, Jiang L, Wang W, Chu H (2020) A novel green reinforcement corrosion inhibitor extracted from waste Platanus acerifolia leaves. Constr Build Mater. https://doi.org/10.1016/j.conbuildmat.2020.119695
Guo X, Wu F, Cheng T, Huang H (2021) Extraction of a high efficiency and long-acting green corrosion inhibitor from silkworm excrement and its adsorption behavior and inhibition mechanism on copper. Colloids Surfaces A Physicochem Eng Asp. https://doi.org/10.1016/j.colsurfa.2021.127679
Milošev I, Bakaric T, Zanna S, Seyeux A, Rodic P, Poberžnik M, Chiter F, Cornette P, Costa D, Kokalj A, Marcus P (2019) Electrochemical, surface-analytical, and computational DFT study of alkaline etched aluminum modified by carboxylic acids for corrosion protection and hydrophobicity. J Electrochem Soc. https://doi.org/10.1149/2.0181911jes
Funding
This study was financially supported by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (Grant No. 451–03-47/2023–01/200135).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Simović, A., Stevanović, S., Milovanović, B. et al. Green corrosion inhibitors of steel based on peptides and their constituents: a combination of experimental and computational approach. J Solid State Electrochem 27, 1821–1834 (2023). https://doi.org/10.1007/s10008-023-05433-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05433-w