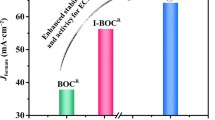
Abstract
The growing energy crisis put an emphasis on the development of novel efficient energy conversion and storage systems. Here we show that surface modification of cobalt by a fast galvanic displacement with rhodium significantly affects the activity towards hydrogen (HER) and oxygen evolution reactions (OER) in alkaline media. After only 20 s of galvanic displacement, the HER overpotential is reduced by 0.16 V and OER overpotential by 0.06 V. This means that the predicted water splitting voltage is reduced from 2.03 V (clean Co anode and cathode) to 1.81 V at 10 mA cm−2 (Rh-exchanged Co electrode). During the galvanic displacement process, the surface roughness of the Co electrode does not suffer significant changes, which suggests an increase in the intrinsic catalytic activity. Density Functional Theory calculations show that the reactivity of the Rh-modified Co(0001) surface is modified compared to that of the clean Co(0001). In the case of HER, experimentally observed activity improvements are directly correlated to the weakening of the hydrogen-surface bond, confirming the beneficial role of Rh incorporation into the Co surface.
Graphical abstract







Similar content being viewed by others
References
Cheng Y, Jiang SP (2015) Advances in electrocatalysts for oxygen evolution reaction of water electrolysis-from metal oxides to carbon nanotubes. Prog Nat Sci Mater Int 25:545–553. https://doi.org/10.1016/j.pnsc.2015.11.008
Esposito D, Chen JG (2011) Monolayer platinum supported on tungsten carbides as low-cost electrocatalysts: opportunities and limitations. Energy Environ Sci 4:3900. https://doi.org/10.1039/c1ee01851e
Rakousky C, Keeley GP, Wippermann K et al (2018) The stability challenge on the pathway to high-current-density polymer electrolyte membrane water electrolyzers. Electrochim Acta 278:324–331. https://doi.org/10.1016/j.electacta.2018.04.154
Gómez JC, Moliner R, Lázaro M (2016) Palladium-based catalysts as electrodes for direct methanol fuel cells: a last ten years review. Catalysts 6:130. https://doi.org/10.3390/catal6090130
Esposito DV, Hunt ST, Kimmel YC, Chen JG (2012) A new class of electrocatalysts for hydrogen production from water electrolysis: metal monolayers supported on low-cost transition metal carbides. J Am Chem Soc 134:3025–3033. https://doi.org/10.1021/ja208656v
Ramaswamy N, Mukerjee S (2011) Influence of inner- and outer-sphere electron transfer mechanisms during electrocatalysis of oxygen reduction in alkaline media. J Phys Chem C 115:18015–18026. https://doi.org/10.1021/jp204680p
Murthy AP, Madhavan J, Murugan K (2018) Recent advances in hydrogen evolution reaction catalysts on carbon/carbon-based supports in acid media. J Power Sources 398:9–26. https://doi.org/10.1016/j.jpowsour.2018.07.040
Wen X, Guan J (2019) Recent progress on MOF-derived electrocatalysts for hydrogen evolution reaction. Appl Mater Today 16:146–168. https://doi.org/10.1016/j.apmt.2019.05.013
Wei J, Zhou M, Long A et al (2018) Heterostructured electrocatalysts for hydrogen evolution reaction under alkaline conditions. Nanomicro Lett. https://doi.org/10.1007/s40820-018-0229-x
Chen Z, Duan X, Wei W et al (2019) Recent advances in transition metal-based electrocatalysts for alkaline hydrogen evolution. J Mater Chem A Mater 7:14971–15005. https://doi.org/10.1039/c9ta03220g
Mohammed-Ibrahim J, Sun X (2019) Recent progress on earth abundant electrocatalysts for hydrogen evolution reaction (HER) in alkaline medium to achieve efficient water splitting. A revew. J Energy Chem 34:111–160. https://doi.org/10.1016/j.jechem.2018.09.016
Gutić S, Dobrota A, Fako E et al (2020) Hydrogen evolution reaction-from single crystal to single atom catalysts. Catalysts 10:290. https://doi.org/10.3390/catal10030290
Fabbri E, Habereder A, Waltar K et al (2014) Developments and perspectives of oxide-based catalysts for the oxygen evolution reaction. Catal Sci Technol 4:3800–3821. https://doi.org/10.1039/c4cy00669k
Butler J (2012) Precious materials handbook. Platin Met Rev 56:267–270. https://doi.org/10.1595/147106712x655357
Li X, Walsh FC, Pletcher D (2011) Nickel based electrocatalysts for oxygen evolution in high current density, alkaline water electrolysers. Phys Chem Chem Phys 13:1162–1167. https://doi.org/10.1039/c0cp00993h
Xu L, Ding Y-S, Chen C-H et al (2007) 3D Flowerlike -nickel hydroxide with enhanced electrochemical activity synthesized by microwave-assisted hydrothermal method. Chem Mater 20:308–316. https://doi.org/10.1021/cm702207w
Smith RDL, Prévot MS, Fagan RD et al (2013) Water oxidation catalysis: electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing iron, cobalt, and nickel. J Am Chem Soc 135:11580–11586. https://doi.org/10.1021/ja403102j
Lyons M, Brandon M (2008) The oxygen evolution reaction on passive oxide covered transition metal electrodes in alkaline solution. Part II - Cobalt. Int J Electrochem Sci 3:1425–1462
Kötz R, Stucki S (1986) Stabilization of RuO2 by IrO2 for anodic oxygen evolution in acid media. Electrochim Acta 31:1311–1316. https://doi.org/10.1016/0013-4686(86)80153-0
Halck NB, Petrykin V, Krtil P, Rossmeisl J (2014) Beyond the volcano limitations in electrocatalysis oxygen evolution reaction. Phys Chem Chem Phys 16:13682–13688. https://doi.org/10.1039/c4cp00571f
Marshall A, Sunde S, Tsypkin M, Tunold R (2007) Performance of a PEM water electrolysis cell using IrxRuyTazO2IrxRuyTazO2 electrocatalysts for the oxygen evolution electrode. Int J Hydrogen Energy 32:2320–2324. https://doi.org/10.1016/j.ijhydene.2007.02.013
Man IC, Su H-Y, Calle-Vallejo F et al (2011) Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3:1159–1165. https://doi.org/10.1002/cctc.201000397
Raabe S, Mierwaldt D, Ciston J et al (2012) In situ electrochemical electron microscopy study of oxygen evolution activity of doped manganite perovskites. Adv Funct Mater 22:3378–3388. https://doi.org/10.1002/adfm.201103173
Jovic B, Lacnjevac U, Jovic V et al (2012) On the kinetics of the hydrogen evolution reaction on Ni-MoOx composite catalysts in alkaline solutions. J Serb Chem Soc 77:211–224. https://doi.org/10.2298/jsc110621185j
Mabayoje O, Liu Y, Wang M et al (2019) Electrodeposition of MoSsubix/i/sub hydrogen evolution catalysts from sulfur-rich precursors. ACS Appl Mater Interfaces 11:32879–32886. https://doi.org/10.1021/acsami.9b07277
Wang Y, Zhang G, Xu W et al (2014) A 3D nanoporous Ni-Mo electrocatalyst with negligible overpotential for alkaline hydrogen evolution. ChemElectroChem 1:1138–1144. https://doi.org/10.1002/celc.201402089
Lačnjevac U, Jović BM, Jović VD (2012) Electrodeposition of Ni, Sn and NiSn alloy coatings from pyrophosphate-glycine bath. J Electrochem Soc 159:D310–D318. https://doi.org/10.1149/2.042205jes
Dimitrov N (2016) Recent advances in the growth of metals, alloys, and multilayers by surface limited redox replacement (SLRR) based approaches. Electrochim Acta 209:599–622. https://doi.org/10.1016/j.electacta.2016.05.115
Papaderakis A, Pliatsikas N, Patsalas P et al (2018) Hydrogen evolution at Ir-Ni bimetallic deposits prepared by galvanic replacement. J Electroanal Chem 808:21–27. https://doi.org/10.1016/j.jelechem.2017.11.055
Ohm D, Domke KF (2021) Controlled deposition of 2D-confined Pd or Irnano-islands on Au(1 1 1) following Cu UPD, and their HER activity. J Electroanal Chem 896:115285. https://doi.org/10.1016/j.jelechem.2021.115285
Brankovic SR, Wang JX, Adžić RR (2001) Metal monolayer deposition by replacement of metal adlayers on electrode surfaces. Surf Sci 474:L173–L179. https://doi.org/10.1016/S0039-6028(00)01103-1
Lačnjevac U, Vasilić R, Dobrota A et al (2020) High-performance hydrogen evolution electrocatalysis using proton-intercalated TiO2 nanotube arrays as interactive supports for Ir nanoparticles. J Mater Chem A Mater 8:22773–22790. https://doi.org/10.1039/D0TA07492F
Jovanović AZ, Bijelić L, Dobrota AS et al (2022) Enhancement of hydrogen evolution reaction kinetics in alkaline media by fast galvanic displacement of nickel with rhodium - from smooth surfaces to electrodeposited nickel foams. Electrochim Acta 414:140214. https://doi.org/10.1016/j.electacta.2022.140214
Łukaszewski M (2016) Electrochemical methods of real surface area determination of noble metal electrodes - an overview. Int J Electrochem Sci 4442–4469. https://doi.org/10.20964/2016.06.71
Trasatti S, Petrii OA (1991) Real surface area measurements in electrochemistry. Pure Appl Chem 63:711–734. https://doi.org/10.1351/pac199163050711
Giannozzi P, Baroni S, Bonini N et al (2009) QUANTUM ESPRESSO: a modular and open-source software project for quantum simulations of materials. J Phys: Condens Matter 21:395502. https://doi.org/10.1088/0953-8984/21/39/395502
Perdew JP, Burke K, Ernzerhof M (1996) Generalized Gradient Approximation made simple. Phys Rev Lett 77:3865–3868. https://doi.org/10.1103/physrevlett.77.3865
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192. https://doi.org/10.1103/physrevb.13.5188
McCrory CCL, Jung S, Ferrer IM et al (2015) Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J Am Chem Soc 137:4347–4357. https://doi.org/10.1021/ja510442p
Brossard L (1992) Cobalt black electrodes for the oxygen evolution reaction from electrolysis of 40 wt% KOH. Int J Hydrogen Energy 17:671–676. https://doi.org/10.1016/0360-3199(92)90085-b
Money Metals. https://www.moneymetals.com/rhodium-price. Accessed 20 Nov 2022
Pašti I, Mentus S (2010) First principles study of adsorption of metals on Pt(111) surface. J Alloys Compd 497:38–45. https://doi.org/10.1016/j.jallcom.2010.03.046
Ruban AV, Skriver HL, Nørskov JK (1999) Surface segregation energies in transition-metal alloys. Phys Rev B 59:15990–16000. https://doi.org/10.1103/physrevb.59.15990
Nørskov JK, Rossmeisl J, Logadottir A et al (2004) Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B 108:17886–17892. https://doi.org/10.1021/jp047349j
Pašti IA, Gavrilov NM, Mentus SV (2011) Hydrogen adsorption on palladium and platinum overlayers: DFT study. Adv Phys Chem 1–8. https://doi.org/10.1155/2011/305634
Zhang J, Tao HB, Kuang M et al (2020) Advances in thermodynamic-kinetic model for analyzing the oxygen evolution reaction. ACS Catal 10:8597–8610. https://doi.org/10.1021/acscatal.0c01906
Huang X, Wang J, Tao HB et al (2019) An essential descriptor for the oxygen evolution reaction on reducible metal oxide surfaces. Chem Sci 10:3340–3345. https://doi.org/10.1039/c8sc04521f
Trasatti S (1972) Work function, electronegativity, and electrochemical behaviour of metals. J Electroanal Chem Interfacial Electrochem 39:163–184. https://doi.org/10.1016/s0022-0728(72)80485-6
Nørskov JK, Bligaard T, Logadottir A et al (2005) Trends in the exchange current for hydrogen evolution. J Electrochem Soc 152:J23. https://doi.org/10.1149/1.1856988
Sheng W, Myint M, Chen JG, Yan Y (2013) Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ Sci 6:1509. https://doi.org/10.1039/c3ee00045a
Gebremariam GK, Jovanović AZ, Dobrota AS et al (2022) Hydrogen Evolution Volcano(es)—From Acidic to Neutral and Alkaline Solutions. Catalysts 12(12):1541. https://doi.org/10.3390/catal12121541
Acknowledgements
The computations and data handling were enabled by resources provided by the Swedish National Infrastructure for Computing (SNIC) at the National Supercomputer Centre (NSC) at Linköping University, partially funded by the Swedish Research Council through grant agreement No. 2018-05973.
Funding
This research was funded by the Science Fund of the Republic of Serbia (PROMIS project RatioCAT), Ministry of Education, Science, and Technological Development of the Republic of Serbia (Contract No. 451–03-68/2022–14/200146). S.V.M. and I.A.P. are indebted to the Research Fund of the Serbian Academy of Sciences and Arts, project F-190, for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nedić Vasiljević, B., Jovanović, A.Z., Mentus, S.V. et al. Galvanic displacement of Co with Rh boosts hydrogen and oxygen evolution reactions in alkaline media. J Solid State Electrochem 27, 1877–1887 (2023). https://doi.org/10.1007/s10008-023-05374-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-023-05374-4