Abstract
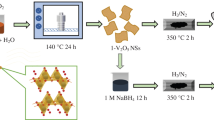
Vanadium oxide film electrodes synthesized by layer-by-layer assembly using sol–gel spin casting and variable 3 & 1 h annealing process over SnO2:F film coated glass substrates are investigated for supercapacitor energy storage using ionic liquid gel-electrolyte. The X-ray photoelectron spectroscopy analysis of V2p3/2 core-level and O1s peak show short-term (1 h) annealing forms vanadium as V2O5 alongside multivalent and oxygen deficient phases, whereas synthesis by 3-h annealing forms stochiometric V2O5 film. In stochiometric V2O5, capacitive contributions are dominantly from redox processes with peaks identified from V5+/V4+, and V4+/V3+ valance states change and partially from electrical double layer (EDL) yielding high specific capacitance of 346.9 Fg−1. Supercapacitor with V2O5 in mixed valence and oxygen defects phases shows enhanced EDL contribution alongside Faradaic yielding 316.2 Fg−1 specific capacitance. These mechanistic differences are analyzed for ionic diffusion limitations. The linear charge/discharge curves at 0.04–0.15 A/g current density show 85–90% Coulomb efficiency and steady energy density 6.8–5.5 Whkg−1 as specific power increases from 1.9 to 5.7 kWkg−1. Oxygen-deficient V2O5-based supercapacitor shows higher 19.2 Whkg−1 energy density declining to 8.9 Whkg−1 with specific power change from 1.1 to 4.6 kWkg−1. Raman spectra show during charge/discharge, the V5+- V3+ redox is mediated by V4.67+, V4.57+, and V3.33+ intermediate valence states. The pseudocapacitive energy storage depends on charge transfer across n+-SnO2:F/n-V2O5 heterostructure with different band alignments for stochiometric and oxygen deficient V2O5. The conduction band shift to the flat band position determines the potential range and extent of V5+ -V3+ redox reaction.














Similar content being viewed by others
References
Yan J, Wang Q, Wei T, Fan Z (2014) Recent advances in design and fabrication of electrochemical supercapacitors with high energy densities. Adv Energy Mater 4:1300816. https://doi.org/10.1002/aenm.201300816
Wu NN, Bai X, Pan D, Dong BB, Wei RB, Naik N, Patil RR, Guo ZH (2021) Recent advances of asymmetric supercapacitors. Adv Mater Interf 8(1):2001710. https://doi.org/10.1002/admi.202001710
Liang R, Du Y, Xiao P, Cheng J, Yuan S, Chen Y, Yuan J, Chen J (2021) Transition metal oxide electrode materials for supercapacitors: A review of recent developments. Nanomaterials 11:1248. https://doi.org/10.3390/nano11051248
Uke SJ, Akhare VP, Bambole DR, Bodade AB, Chaudhari GN (2017) Recent advancements in the cobalt oxides, manganese oxides and their composite as an electrode material for supercapacitor A review. Front Mater 4: Article Number 21. https://doi.org/10.3389/fmats.2017.00021
Lu W, Li Y, Yang M, Jiang X, Zhang YX, Xing Y (2020) Construction of hierarchical Mn2O3@MnO2 core–shell nanofibers for enhanced performance supercapacitor electrodes. ACS Appl Energy Mater 3:8190–8197. https://doi.org/10.1021/acsaem.0c00392
Chen DD, Li, JF, Wu QS (2019) Review of V2O5-based nanomaterials as electrode for supercapacitor. J Nanoparticle Res 21(9): Article 201 https://doi.org/10.1007/s11051-019-4645-8
Zhang Y, Zhao Y, Cao S, Yin Z, Cheng L, Wu L (2017) Design and synthesis of hierarchical SiO2@C/TiO2 hollow spheres for high-performance supercapacitors. ACS Appl Mater Interf 9(35):29982–29991. https://doi.org/10.1021/acsami.7b08776
Hong WL, Lin LY (2019) Studying the substrate effects on energy storage abilities of flexible battery supercapacitor hybrids based on nickel cobalt oxide and nickel cobalt oxide@nickel molybdenum oxide. Electrochim Acta 308:83–90. https://doi.org/10.1016/j.electacta.2019.04.023
Arul CA, Ravi V, Gobalakrishnan VG, Rengasamy S (2021) Electrochromic behavior of vanadium pentoxide thin films prepared by a sol–gel spin coating process. Physica Stat Sol 218(19):2100282. https://doi.org/10.1002/pssa.202100282
Zhang L, Jiang C, Wu C, Ju H, Jiang G, Liu W, Zhu CF, Chen T (2018) V2O5 as hole transporting material for efficient all inorganic Sb2S3 solar cells. ACS Appl Mater Interf 10(32):27098–27105. https://doi.org/10.1021/acsami.8b09843
Sun M, Taha M, Walia S, Bhaskaran M, Sriram S, Shieh W, Unnithan RR (2018) A photonic switch based on a hybrid combination of metallic nanoholes and phase-change vanadium dioxide. Sci Rep 8: Article 11106. https://doi.org/10.1038/s41598-018-29476-6
Allabergenov B, Yun S, Cho H-S, Lyu H-K, Choi B (2021) Control of Polymorphic Properties of Multivalent Vanadium Oxide Thin Films. ACS Appl Electron Mater 3(3):1142–1150. https://doi.org/10.1021/acsaelm.0c01010
Becker M, Kessler J, Kuhl F, Benz SL, Chen L, Polity A, Klar PJ, Chatterjee S (2022) Phase control of multivalent vanadium oxides VOx by ion-beam sputter-deposition. Physica Stat Solidi 219(9):2100828. https://doi.org/10.1002/pssa.202100828
Zhi M, Xiang C, Li J, Li M, Wu N (2013) Nanostructured carbon–metal oxide composite electrodes for supercapacitors:a review. Nanoscale 5:72–88
Pan AQ, Wu HB, Zhang L, Lou XW (2013) Uniform V2O5 nanosheet-assembled hollow microflowers with excellent lithium storage properties. Energy Environ Sci 6:1476–1479. https://doi.org/10.1039/c3ee40260f
Hua L, Ma ZY, Shi PP, Li L, Rui K, Zhou JY, Huang X, Liu X, Zhu JX, Sun GZ, Huang W (2017) Ultrathin and large-sized vanadium oxide nanosheets mildly prepared at room temperature for high performance fiber-based supercapacitors. J Mater Chem A 5:2483–2487. https://doi.org/10.1039/C6TA10619F
Li HY, Wei C, Wang L, Zuo QS, Li X, Xie B (2015) Hierarchical vanadium oxide microspheres forming from hyperbranched nanoribbons as remarkably high-performance electrode materials for supercapacitors. J Mater Chem 3(45):22892–22901. https://doi.org/10.1039/c5ta06088e
Kiruthiga R, Nithya C, Karvembu R (2017) Reduced graphene oxide embedded V2O5 nanorods and porous honey carbon as high-performance electrodes for hybrid sodium-ion supercapacitors. Electrochim Acta 256:221–231. https://doi.org/10.1016/j.electacta.2017.10.049
Kianfa E (2019) Recent advances I n synthesis, properties, and applications of vanadium oxide nanotube. Microchem J 145:966–978. https://doi.org/10.1016/j.microc.2018.12.008
Yao L, Zhang C, Hu N, Zhang L, Zhou Z, Zhang Y (2019) Three-dimensional skeleton networks of reduced graphene oxide nanosheets/vanadium pentoxide nanobelts hybrid for high-performance supercapacitors. Electrochim Acta 295:14–21. https://doi.org/10.1016/j.electacta.2018.10.134
Zheng K, Zeng YX, Lu XH, Zeng ZK, Y, Liu S, Zeng C, Tong Y, Zheng Z, Zhu T, Lu X (2019) Valence and surface modulated vanadium oxide nanowires as new high-energy and durable negative electrode for flexible asymmetric supercapacitors. Energy Storage Mater 22:410–417. https://doi.org/10.1016/j.ensm.2019.02.012
Tian M, Li R, Liu C, Long D, Cao G (2019) Aqueous Al-Ion Supercapacitor with V2O5 Mesoporous Carbon Electrodes. ACS Appl Mater Interf 11:15573–15580. https://doi.org/10.1021/acsami.9b02030
Wu Y, Gao G, Yang H, Bi W, Liang X, Zhang Y, Zhang G, Wu G (2015) Controlled synthesis of V2O5/MWCNT core/shell hybrid aerogels through a mixed growth and self-assembly methodology for supercapacitors with high capacitance and ultralong cycle life. J Mater Chem A 3:15692–15699. https://doi.org/10.1039/C5TA02708J
Yilmaz G, Lu X, Ho G (2017) Cross-linker mediated formation of sulfur-functionalized V2O5/graphene aerogels and their enhanced pseudocapacitive performance. Nanoscale 7(9):802–811. https://doi.org/10.1039/C6NR08233E
Choudhury A, Bonso J, Wunch M, Yang K, Ferraris J, Yang D (2015) In-situ synthesis of vanadium pentoxide nanofibre/exfoliated graphene nanohybrid and its supercapacitor applications. J Power Sources 87:283–290. https://doi.org/10.1016/j.jpowsour.2015.04.062
Qin H, Liang S, Chen L, Li Y, Luo Z, Chen S (2020) Recent advances in vanadium-based nanomaterials and their composites for supercapacitors. Sustainable Energy Fuels 4:4902–4933. https://doi.org/10.1039/D0SE00897D
Kim A, Kalita G, Kim JH, Patel R (2021) Recent development in vanadium pentoxide and carbon hybrid active materials for energy storage devices. Nanomaterials 11(12):3213. https://doi.org/10.3390/nano11123213
Montoux A, Groult H, Balnois E, Doppelt P, Gueroudji L (2004) Vanadium oxide films synthesized by CVD and used as positive electrodes in secondary lithium batteries. J Electrochem Soc 151(3):A368. https://doi.org/10.1149/1.1641037
Lv H, Pan Q, Song Y, Liu X-X, Liu T (2020) A review on nano-/microstructured materials constructed by electrochemical technologies for supercapacitors. Nano-Micro Lett 12:Article 118. https://doi.org/10.1007/s40820-020-00451-z
Gandla D, Tan DQ (2019) Progress report on atomic layer deposition toward hybrid nanocomposite electrodes for next generation supercapacitors. Adv Mater Interf 6(16):1900678. https://doi.org/10.1002/admi.201900678
Zilberberg K, Trost S, Meyer J, Kahn A, Behrendt A, Lutzenkirchen-Hecht D, Frahm R, Riedl T (2011) Inverted organic solar cells with sol–gel processed high work-function vanadium oxide hole-extraction layers. Adv Funct Mater 21:4776–4783. https://doi.org/10.1002/adfm.201101402
Schneider K (2020) Optical properties and electronic structure of V2O5, V2O3 and VO2. J Mater Sci Mater Electronics 31:10478–10488. https://doi.org/10.1007/s10854-020-03596-0
Engstrom AM, Doyle FM (2013) Exploring the cycle behavior of electrodeposited vanadium oxide electrochemical electrode in various aqueous environments. J Power Sources 228:120–131. https://doi.org/10.1016/j.jpowsour.2012.11.075
Craig J, Fontenot JW, Wiench MP, Schrader GL (2000) Vanadia gel synthesis via peroxovanadate precursors: In situ laser Raman and 51V NMR characterization of the gelation process. J Phys Chem B 104:11622–11631. https://doi.org/10.1021/jp0021897
Liu Y, Clark M, Zhang Q, Yu D, Liu D, Liu J, Cao G (2011) V2O5 nano-electrodes with high power and energy densities for thin film li-ion batteries. Adv Energy Mater 1:194–202. https://doi.org/10.1002/aenm.201000037
Liu H, Yang W (2011) Ultralong single crystalline V2O5 nanowire/graphene composite fabricated by a facile green approach and its lithium storage behavior. Energy Environ Sci 4:4000–4008. https://doi.org/10.1039/C1EE01353J
Monfort O, Petrisková P (2021) Binary and ternary vanadium oxides:general overview, physical properties, and photochemical processes for environmental applications. Processes 9:214. https://doi.org/10.3390/pr9020214
Biesinger MC, Lau LWM, Gerson AR, Smart RS (2010) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides:Sc, V, Cu, Zn. Appl Surf Sci 257:887–898. https://doi.org/10.1016/j.apsusc.2010.07.086
Silversmit G, Depla D, Poelman H, Marin G, De Gryse R (2004) Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J Electron Spectros Relat Phenomena 135(2–3):167–175. https://doi.org/10.1016/j.elspec.2004.03.004
NIST X-ray Photoelectron Spectroscopy Database, NIST Standard Reference Database Number 20, National Institute of Standards and Technology, Gaithersburg MD, 20899 (2000). https://doi.org/10.18434/T4T88K
Urena-Begara F, Crunteanu A, Raskin J (2017) Raman and XPS characterization of vanadium oxide thin films with temperature. Appl Surf Sci 403:717–727. https://doi.org/10.1016/j.apsusc.2017.01.160
Perera SD, Patel B, Nijem N, Roodenko K, Seitz O, Ferraris JP, Chabal YJ, Balkus KJ Jr (2011) Vanadium oxide nanowire-carbon nanotube binder-free flexible electrodes for supercapacitors. Adv Energy Mater 1(5):936–945. https://doi.org/10.1002/aenm.201100221
Światowska-Mrowiecka J, Maurice V, Zanna S, Klein L, Marcus P (2007) XPS study of Li ion intercalation in V2O5 thin films prepared by thermal oxidation of vanadium metal. Electrochim Acta 52(18):5644–5653. https://doi.org/10.1016/j.electacta.2006.12.050
Sathiya M, Prakash AS, Ramesha K, Tarascon J, Shukla AK (2011) V2O5-anchored carbon nanotubes for enhanced electrochemical energy storage. J Am Chem Soc 133(40):16291–16301. https://doi.org/10.1021/ja207285b
Antonides E, Janse EC, Sawatzky GA (1977) LMM Auger spectra of Cu, Zn, Ga, and Ge. II. Relationship with L23 photoelectron spectra via the L2L3M45 Coster-Kronig process. Phys Rev B 15(10)4596–4601. https://doi.org/10.1103/PhysRevB.15.4596
Demeter M, Neumann M, Reichelt W (2000) Mixed-valence vanadium oxides studied by XPS. Surf Sci 454–456:41–44. https://doi.org/10.1016/S0039-6028(00)00111-4
Wu Q, Thissen A, Jaegermann W, Liu M (2004) Photoelectron spectroscopy study of oxygen vacancy on vanadium oxides surface. Appl Surf Sci 236(1–4):473–478. https://doi.org/10.1016/j.apsusc.2004.05.112
Sawatzky GA, Post D (1979) X-ray photoelectron and Auger spectroscopy study of some vanadium oxides. Phys Rev B 20(4):1546–1555. https://doi.org/10.1103/PhysRevB.20.1546
Ramana CV, Hussain OM, Naidu BS, Reddy PJ (1997) Spectroscopic characterization of electron-beam evaporated V2O5 thin films. Thin Solid Films 305(1–2):219–226. https://doi.org/10.1016/S0040-6090(97)00141-7
Yan J, Liu J, Fan Z, Wei T, Zhang L (2012) High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon 50(6):2179–2188. https://doi.org/10.1016/j.carbon.2012.01.028
Chen Z, Qin Y, Weng D, Xiao Q, Peng Y, Wang X, Li H, Wei F, Lu Y (2009) Design and synthesis of hierarchical nanowire composites for electrochemical energy storage. Adv Funct Mater 19(21):3420–3426. https://doi.org/10.1002/adfm.200900971
Bi W, Wu Y, Liu C, Wang J, Du Y, Gao G, Wu G, Cao G (2019) Gradient oxygen vacancies in V2O5/PEDOT nanocables for high-performance supercapacitors. ACS Appl Energy Mater 2(1):668–677. https://doi.org/10.1021/acsaem.8b01676
Augustyn V, Simon P, Dunn B (2014) Pseudocapacitive oxide for high-rate electrochemical energy storage. Energy Environ Sci 7:1597–1614. https://doi.org/10.1039/C3EE44164D
Wang J, Polleux J, Lim J, Dunn B (2007) Pseudocapacitive contributions to electrochemical energy storage in TiO2 (Anatase) nanoparticles. J Phys Chem C 111:14925–14931. https://doi.org/10.1021/jp074464w
Baronetto D, Krstajic N, Trasatti S (1994) Reply to “note on a method to interrelate inner and outer electrode areas” By H. Vogt Electrochim Acta 39(16):2359–2362. https://doi.org/10.1016/0013-4686(94)E0158-K
Nakayama M, Kanaya T, Inoue R (2007) Anodic deposition of layered manganese oxide into a colloidal crystal template for electrochemical supercapacitor. Electrochem Commun 9(5):1154–1158. https://doi.org/10.1016/j.elecom.2007.01.021
Sharma RK, Rastogi AC, Desu SB (2008) Manganese oxide embedded polypyrrole nanocomposites for electrochemical supercapacitor. Electrochim Acta 53:7690–7695. https://doi.org/10.1016/j.electacta.2008.04.028
Shvets P, Dikaya O, Maksimova K, Goikhman A (2019) A review of Raman spectroscopy of vanadium oxides. J Raman Spectrosc 50(8):1226–1244. https://doi.org/10.1002/jrs.5616
Baddour-Hadjean R, Smirnov MB, Smirnov KS, Kazimirov V, Gallardo-Amores JM, Amador U, Dompablo ME, Pereir-Ramos JP (2012) Lattice Dynamics of β-V2O5: Raman Spectroscopic Insight into the Atomistic Structure of a High-Pressure Vanadium Pentoxide Polymorph. Inorg Chem 51(5):3194–3201. https://doi.org/10.1021/ic202651b
Chen W, Mai L, Peng J, Xu Q, Zhu Q (2003) Raman spectroscopic study of vanadium oxide nanotubes. J Solid State Chem 177:377–379. https://doi.org/10.1016/S0022-4596(03)00416-X
Sieradzka K, Wojcieszak D, Kaczmarek D, Kiriakidis G, Aperathitis E, Kambilafka V, Placido F, Song S (2011) Structural and optical properties of vanadium oxides prepared by microwave-assisted reactive magnetron sputtering. Opt Appl 41(2):463–469
Baddour-Hadjean R, Smirnov MB, Kazimirov VY, Smirnov KS, Pereira-Ramos J (2015) The Raman spectrum of the γ′-V2O5 polymorph:a combined experimental and DFT study. J Raman Spectrosc 46:406–412. https://doi.org/10.1002/jrs.4660
Tatsuyama C, Fan HY (1980) Raman scattering and phase transitions in V2O3 and (V1–x Crx)2O3. Phys Rev B 21:2977–2983
Kuroda N, Fan HY (1977) Raman scattering and phase transitions of V2O3. Phys Rev B 16:5003–5008
Mei M, Liu J, Zhong H, Wang S, Li Z-f, Chen X, Lu W (2004) Raman study of the phase transition in VO2 thin films. J Cryst Growth 268(1–2):178–183
Petrov GI, Yakovlev VV (2002) Raman microscopy analysis of phase transformation mechanisms in vanadium dioxide. Appl Phys Lett 81(6):1023–1025. https://doi.org/10.1063/1.1496506
Yuan X, Zhang W, Zhang P (2013) Hole-lattice coupling and photoinduced insulator-metal transition in VO2. Phys Rev B 88(3):035119. https://doi.org/10.1103/PhysRevB.88.035119
Shibuya K, Sawa A (2017) Polarized Raman scattering of epitaxial vanadium dioxide films with low-temperature monoclinic phase. J Appl Phys 122(1):015307. https://doi.org/10.1063/1.4990988
Berenguer R, Guerrero-Pérez MO, Guzmán I, Rodríguez-Mirasol J, Cordero T (2017) Synthesis of vanadium oxide nanofibers with variable crystallinity and V5+/V4+ ratios. ACS Omega 2(11):7739–7745. https://doi.org/10.1021/acsomega.7b01061
Souza Filho AG, Ferreira OP, Santos EJG, Mendes Filho J, Alves OL (2004) Raman spectra in vanadate nanotubes revisited. Nano Lett 4(11):2099–2104. https://doi.org/10.1021/nl0488477
Julien C, Nazri GA, Bergström O (1997) Raman scattering studies of microcrystalline V6O13. Phy Status Solidi B 201:319–326. https://doi.org/10.1002/1521-3951(199705)201:1%3c319:AID-PSSB319%3e3.3.CO;2-K
Meyer J, Zilberberg K, Riedl T, Kahn A (2011) Electronic structure of vanadium pentoxide:an efficient hole injector organic electronic material. J Appl Phys 110:033710. https://doi.org/10.1063/1.3611392
Wang ZB, Helander MG, Qiu J, Liu ZW, Greiner MT, Lu ZH (2010) Direct hole injection in to 4, 4’-N, N’-dicarbazole-biphenyl:a simple pathway to achieve efficient organic light emitting diodes. J Appl Phys 108:024510. https://doi.org/10.1063/1.3456513
Zhang HM, Wallace CH (2008) Indium tin oxide modified by Au and vanadium pentoxide as an efficient anode for organic light-emitting devices. IEEE Trans Electron Devices 55(9):2517–2520. https://doi.org/10.1109/TED.2008.927387
Wang F, Tan Z, Li Y (2015) Solution-processable metal oxides/chelates as electrode buffer layers for efficient and stable polymer solar cells. Energy Environ Sci 8:1059–1091. https://doi.org/10.1039/C4EE03802A
Xie F, Choy WCH (2013) Hydrogen metal oxide bronzes for efficient hole transport layers. SPIE Newsroom. https://doi.org/10.1117/2.1201311.005133
Chen X, Zhu K, Wang P, Sun G, Yao Y, Luo W, Zou Z (2020) Reversible Charge Transfer and Adjustable Potential Window in Semiconductor/Faradaic Layer/Liquid Junctions. iScience 23:100949. http://creativecommons.org/licenses/by-nc-nd/4.0/
Acknowledgements
The authors acknowledge the grant ADLG-181 from Analytical and Diagnostics Laboratory (ADL), a part of Center of Excellence in Small Scale Integration and Packaging at Binghamton University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Azadian, F., Rastogi, A.C. Electrochemical and energy storage properties of layer-by-layer assembled vanadium oxide electrode-based solid-state supercapacitor in n+-SnO2:F/n-V2O5 heterostructure device form using ionic liquid gel electrolyte. J Solid State Electrochem 27, 139–159 (2023). https://doi.org/10.1007/s10008-022-05309-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05309-5