Abstract
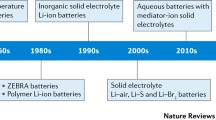
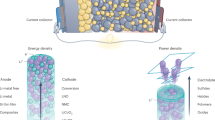
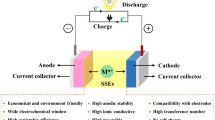
As battery technologies are in continuous development, and especially due to the rapid growth in vehicle electrification, which requires large (e.g., 100 s of kg) battery packs, there has been a growing demand for more efficient, reliable, and environmentally friendly materials. Solid-state post-lithium-ion batteries are considered a possible next-generation energy storage technology. One immediate advantage of these power sources over commercial lithium-ion batteries is the potential of solving the resource issues facing LIBs, especially as cost-effective alternatives. The second advantage is the removal of flammable liquid electrolytes. The solid electrolytes are more resistant to changes in temperature and physical damage, produce up to 80% less heat, and are able to handle more charge/discharge cycles before degradation makes them unusable. All these features point towards a longer battery life. Other immediate gains include the removal of the membrane and casing required for a liquid electrolyte. This may reduce the weight and volume of each cell, leading to an increase in the energy density of the battery. In this review, we describe recent achievements in the development of sodium, potassium, and magnesium solid-state batteries. It can be revealed that while the research community has progressed greatly towards solid-state alkali and alkaline-earth batteries, much more improvement in the room temperature ionic conductivity of solid electrolytes is required. For the practical applications of these systems, the stability and interfacial reactions of solid electrolytes should be explored in great depth.










Similar content being viewed by others
References
Verma R, Didwal PN, Hwang JY, Park CJ (2021) Recent progress in electrolyte development and design strategies for next-generation potassium-ion batteries. Batter Supercaps 4(9):1428–1450. https://doi.org/10.1002/BATT.202100029
Aurbach D et al (2000) Prototype systems for rechargeable magnesium batteries. Nature 407(6805):724–727. https://doi.org/10.1038/35037553
Yoo HD, Shterenberg I, Gofer Y, Gershinsky G, Pour N, Aurbach D (2013) Mg rechargeable batteries: an on-going challenge. Energy Environ Sci 6(8):2265–2279. https://doi.org/10.1039/c3ee40871j
Song J, Sahadeo E, Noked M, Lee SB (2016) Mapping the challenges of magnesium battery. J Phys Chem Lett 7(9). https://doi.org/10.1021/acs.jpclett.6b00384
Sun C, Liu J, Gong Y, Wilkinson DP, Zhang J (2017) Recent advances in all-solid-state rechargeable lithium batteries. Nano Energy 33:363–386. https://doi.org/10.1016/J.NANOEN.2017.01.028
Chen R, Li Q, Yu X, Chen L, Li H (2019) Approaching practically accessible solid-state batteries: stability issues related to solid electrolytes and interfaces. Chem Rev 120(14):6820–6877. https://doi.org/10.1021/ACS.CHEMREV.9B00268
Randau S et al (2020) Benchmarking the performance of all-solid-state lithium batteries. Nat Energy 5(3):259–270 . https://doi.org/10.1038/s41560-020-0565-1
Funke K (1993) Jump relaxation in solid ionic conductors. Solid St Chem 22:111–195
Li Z et al (2021) Solid-state electrolytes for sodium metal batteries. Energy Fuels 35(11):9063–9079. https://doi.org/10.1021/ACS.ENERGYFUELS.1C00347
Leyet Y et al (2018) Synthesis of Na2Ti3O7 nanoparticles by sonochemical method for solid state electrolyte applications. J Solid State Electr 22(5):1315–1319. https://doi.org/10.1007/s10008-017-3697-x
Kusnezoff M, Wagner D, Schilm J, Heubner C, Matthey B, Lee CW (2022) Influence of microstructure and crystalline phases on impedance spectra of sodium conducting glass ceramics produced from glass powder. J Solid State Electrochem 26(2):375–388. https://doi.org/10.1007/S10008-021-05063-0/TABLES/5
Yang K, Liu D, Qian Z, Jiang D, Wang R (2021) Computational auxiliary for the progress of sodium-ion solid-state electrolytes. ACS Nano 15(11):17232–17246. https://doi.org/10.1021/ACSNANO.1C07476/ASSET/IMAGES/ACSNANO.1C07476.SOCIAL.JPEG_V03
Zhao C et al (2018) Solid-state sodium batteries sodium-ion batteries view project inorganic crystal compounds view project solid-state sodium batteries. https://doi.org/10.1002/aenm.201703012
Yue J et al (2017) High-performance all-inorganic solid-state sodium-sulfur battery. ACS Nano 11(5):4885–4891. https://doi.org/10.1021/ACSNANO.7B01445
Lu Y, Li L, Zhang Q, Niu Z, Chen J (2018) Electrolyte and interface engineering for solid-state sodium batteries. Joule 2(9):1747–1770. https://doi.org/10.1016/J.JOULE.2018.07.028
Hou W, Guo X, Shen X, Amine K, Yu H, Lu J (2018) Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective. Nano Energy 52:279–291. https://doi.org/10.1016/j.nanoen.2018.07.036
Wang E, Greenblatt M (1991) Ionic conductivity of potassium phosphatoantimonates and some of their ion-exchanged analogues. Chem Mater 3(3):542–546. https://doi.org/10.1021/CM00015A034
Wang E, Greenblatt M (1991) Ionic conductivities of ion-exchanged A3Sb3P2O14(A = Na, K, Rb), A5Sb5P2O20(A = Li, Na, K, Rb), and partially substituted K5Sb5–xMxP2O20(M = Nb, Ta). Chem Mater 3(4):703–709. https://doi.org/10.1021/CM00016A026
Doux JM, Leguay L, Le Gal La A, Salle OJ, Quarez E (2018) K3Sb4O10(BO3): a solid state K-ion conductor. Solid State Ionics 324:260–266. https://doi.org/10.1016/j.ssi.2018.07.018
Yuan H et al (2018) A K2Fe4O7 superionic conductor for all-solid-state potassium metal batteries. J Mater Chem A 6(18):8413–8418. https://doi.org/10.1039/C8TA01418C
Zheng J, Fang H, Fan L, Ren Y, Jena P, Wu Y (2021) Antiperovskite K3OI for K-ion solid state electrolyte. J Phys Chem Lett 12(30):7120–7126. https://doi.org/10.1021/ACS.JPCLETT.1C01807/SUPPL_FILE/JZ1C01807_SI_001.PDF
Xiao R, Li H, Chen L (2020) High-throughput computational discovery of K2CdO2 as an ion conductor for solid-state potassium-ion batteries. J Mater Chem A 8(10):5157–5162. https://doi.org/10.1039/C9TA13105A
Haffner A et al (2021) Polymorphism and fast potassium-ion conduction in the T5 supertetrahedral phosphidosilicate KSi2P3. Angew Chemie Int Ed 60(24):13641–13646. https://doi.org/10.1002/ANIE.202101187
Zhan Y, Zhang W, Lei B, Liu H, Li W (2020) Recent development of Mg ion solid electrolyte. Front Chem 8(February):1–7. https://doi.org/10.3389/fchem.2020.00125
Jaschin PW, Gao Y, Li Y, Bo SH (2020) A materials perspective on magnesium-ion-based solid-state electrolytes. J Mater Chem A 8(6):2875–2897. https://doi.org/10.1039/c9ta11729f
Ikeda S, Takahashi M, Ishikawa J, Ito K (1987) Solid electrolytes with multivalent cation conduction. 1. Conducting species in MgZrPO4 system. Solid State Ionics 23(1–2):125–129. https://doi.org/10.1016/0167-2738(87)90091-9
Kazakos-Kijowski A, Komarneni S, Agrawal D, Roy R (1988) Synthesis, crystal data and thermal stability of magnesium zirconium phosphate [MgZr4(PO4)6]. Mater Res Bull 23(8):1177–1184. https://doi.org/10.1016/0025-5408(88)90209-7
Nakano K, Noda Y, Tanibata N, Nakayama M, Kajihara K, Kanamura K (2019) RSC advances computational investigation of the Mg-ion. 4:12590–12595. https://doi.org/10.1039/c9ra00513g
Matsuo M et al (2013) Sodium and magnesium ionic conduction in complex hydrides. J Alloys Compd 580:S98–S101. https://doi.org/10.1016/j.jallcom.2013.01.058
Higashi S, Miwa K, Aoki M, Takechi K (2014) A novel inorganic solid state ion conductor for rechargeable Mg batteries. Chem Commun 50(11):1320–1322. https://doi.org/10.1039/c3cc47097k
Canepa P et al (2017) High magnesium mobility in ternary spinel chalcogenides. Nat Commun 8(1):1–8. https://doi.org/10.1038/s41467-017-01772-1
Canepa P, Sai Gautam G, Broberg D, Bo SH, Ceder G (2017) Role of point defects in spinel Mg chalcogenide conductors. Chem Mater 29(22):9657–9667. https://doi.org/10.1021/acs.chemmater.7b02909
Chen T, Sai Gautam G, Canepa P (2019) Ionic transport in potential coating materials for Mg batteries. Chem. Mater 31(19):8087–8099. https://doi.org/10.1021/acs.chemmater.9b02692
Kundu S, Solomatin N, Kauffmann Y, Kraytsberg A, Ein-Eli Y (2021) Revealing and excluding the root cause of the electronic conductivity in Mg-ion MgSc2Se4 solid electrolyte. Appl Mater Today 23:100998. https://doi.org/10.1016/J.APMT.2021.100998
Fenton DE, Parker JM, Wright PV (1973) Complexes of alkali metal ions with poly(ethylene oxide). Polymer (Guildf) 14(11):589. https://doi.org/10.1016/0032-3861(73)90146-8
Armand MB, Chabagno JM, Duclot MJ (1979) Polyethers as solid electrolytes. Fast Ion Transp Solids Electrodes Electrolytes Proc Int Conf 131–136
Armand MB, Bruce PG, Forsyth M, Scrosati B, Wieczorek W (2011) Polymer electrolytes. In: Bruce DW, O’Hare D, Walton RI (eds) Energy Materials. Chichester, UK, John Wiley & Sons, Ltd, pp 1–31
Chen R, Qu W, Guo X, Li L, Wu F (2016) The pursuit of solid-state electrolytes for lithium batteries: from comprehensive insight to emerging horizons. Mater Horiz 3(6):487–516. https://doi.org/10.1039/C6MH00218H
Hallinan DT, Balsara NP (2013) Polymer electrolytes. Annual Review of Materials Research. 43. Ann Rev 503–525. https://doi.org/10.1146/annurev-matsci-071312-121705
Bocharova V, Sokolov AP (2020) Perspectives for polymer electrolytes: a view from fundamentals of ionic conductivity. Macromolecules 53(11):4141–4157. https://doi.org/10.1021/acs.macromol.9b02742
Bresser D, Lyonnard S, Iojoiu C, Picard L, Passerini S (2019) Decoupling segmental relaxation and ionic conductivity for lithium-ion polymer electrolytes. Molecular Systems Design and Engineering 4(4):779–792. https://doi.org/10.1039/c9me00038k
Ratner MA, Johansson P, Shriver DF (2000) Polymer electrolytes: ionic transport mechanisms and relaxation coupling. MRS Bull 25(3):31–37. https://doi.org/10.1557/MRS2000.16
Golodnitsky D, Strauss E, Peled E, Greenbaum S (2015) Review—On order and disorder in polymer electrolytes. J Electrochem Soc 162(14):A2551–A2566. https://doi.org/10.1149/2.0161514jes
Croce F, Persi L, Scrosati B, Serraino-Fiory F, Plichta E, Hendrickson MA (2001) Role of the ceramic fillers in enhancing the transport properties of composite polymer electrolytes. Electrochim Acta 46:2457–2461
Bonizzoni S, Ferrara C, Berbenni V, Anselmi-Tamburini U, Mustarelli P, Tealdi C (2019) NASICON-type polymer-in-ceramic composite electrolytes for lithium batteries. Phys Chem Chem Phys 21(11):6142–6149. https://doi.org/10.1039/C9CP00405J
Blanga R, Burstein L, Berman M, Greenbaum SG, Golodnitsky D (2015) Solid polymer-in-ceramic electrolyte formed by electrophoretic deposition. J Electrochem Soc 162(11):D3084–D3089. https://doi.org/10.1149/2.0221511jes
Chen L, Li Y, Li SP, Fan LZ, Nan CW, Goodenough JB (2018) PEO/garnet composite electrolytes for solid-state lithium batteries: from ‘ceramic-in-polymer’ to ‘polymer-in-ceramic.’ Nano Energy 46:176–184. https://doi.org/10.1016/J.NANOEN.2017.12.037
Menkin S et al (2019) Evaluation of ion-transport in composite polymer-in-ceramic electrolytes. Case study of active and inert ceramics. Electrochim Acta 304:447–455. https://doi.org/10.1016/j.electacta.2019.03.006
Blanga R et al (2013) Quasi-solid polymer-in-ceramic membrane for Li-ion batteries. Electrochim Acta 114:325–333. https://doi.org/10.1016/j.electacta.2013.09.106
Bae J et al (2018) A 3D nanostructured hydrogel-framework-derived high-performance composite polymer lithium-ion electrolyte. Angew Chemie - Int Ed 57(8):2096–2100. https://doi.org/10.1002/anie.201710841
Peled E, Golodnitsky D (2004) SEI on lithium, graphite, disordered carbons and tin-based alloys. Lithium-Ion Batter 1–69. https://doi.org/10.1142/9781860946448_0001
Watanabe M, Thomas ML, Zhang S, Ueno K, Yasuda T, Dokko K (2017) Application of ionic liquids to energy storage and conversion materials and devices. Chem Rev 117(10):7190–7239. https://doi.org/10.1021/ACS.CHEMREV.6B00504
Song S et al (2021) Highly conductive ionogel electrolytes based on N-ethyl-N-methylpyrrolidinium bis(fluorosulfonyl)imide FSI and NaFSI mixtures and their applications in sodium batteries. J Phys Mater 4(4):044005. https://doi.org/10.1088/2515-7639/AC0800
Qi X et al (2016) Sodium bis(fluorosulfonyl)imide/poly(ethylene oxide) polymer electrolytes for sodium-ion batteries. ChemElectroChem 3(11):1741–1745. https://doi.org/10.1002/CELC.201600221
Ma Q et al (2017) A new Na[(FSO2)(n-C4F9SO2)N]-based polymer electrolyte for solid-state sodium batteries. J Mater Chem A 5(17):7738–7743. https://doi.org/10.1039/C7TA01820G
Moreno JS, Armand M, Berman MB, Greenbaum SG, Scrosati B, Panero S (2014) Composite PEOn:NaTFSI polymer electrolyte: preparation, thermal and electrochemical characterization. J Power Sources 248:695–702. https://doi.org/10.1016/J.JPOWSOUR.2013.09.137
Ponrouch A, Monti D, Boschin A, Steen B, Johansson P, Palacín MR (2014) Non-aqueous electrolytes for sodium-ion batteries. J Mater Chem A 3(1):22–42. https://doi.org/10.1039/C4TA04428B
Peta G et al (2021) Toward high performance all solid-state Na batteries: investigation of electrolytes comprising NaPF6, poly(ethylene oxide) and TiO2. J Electrochem Soc 168(11):110553. https://doi.org/10.1149/1945-7111/AC330D
Arya A, Sharma AL (2018) Optimization of salt concentration and explanation of two peak percolation in blend solid polymer nanocomposite films. J Solid State Electr 22(9):2725–2745. https://doi.org/10.1007/s10008-018-3965-4
Mokhtar M, Majlan EH, Talib MZM, Ahmad A, Tasirin SM, Daud WRW (2016) A short review on alkaline solid polymer electrolyte based on polyvinyl alcohol (PVA) as polymer electrolyte for electrochemical devices applications. Int J Appl Eng Res 11(19):10009–10015. Research India Publications
Osman Z, Isa KB, Ahmad A, Othman L (2010) A comparative study of lithium and sodium salts in PAN-based ion conducting polymer electrolytes. Ionics (Kiel) 16(5):431–435. https://doi.org/10.1007/S11581-009-0410-9/FIGURES/5
Mishra K, Arif T, Kumar R, Kumar D (2019) Effect of Al2O3 nanoparticles on ionic conductivity of PVdF-HFP/PMMA blend-based Na+-ion conducting nanocomposite gel polymer electrolyte. J Solid State Electrochem 23(8):2401–2409. https://doi.org/10.1007/S10008-019-04348-9
Singh VK et al (2018) Electrochemical investigations of Na0.7CoO2 cathode with peo-natfsi-bmimtfsi electrolyte as promising material for na-rechargeable battery. J Solid State Electrochem 22(6):1909–1919. https://doi.org/10.1007/S10008-018-3891-5
Diana MI, Selvin PC, Selvasekarapandian S, Krishna MV (2021) Investigations on Na-ion conducting electrolyte based on sodium alginate biopolymer for all-solid-state sodium-ion batteries. J Solid State Electrochem 25(7):2009–2020. https://doi.org/10.1007/S10008-021-04985-Z
Reddy CVS, Sharma AK, Narasimha Rao VVR (2006) Electrical and optical properties of a polyblend electrolyte. Polymer (Guildf) 47(4):1318–1323. https://doi.org/10.1016/J.POLYMER.2005.12.052
Fei H et al (2018) Stable all-solid-state potassium battery operating at room temperature with a composite polymer electrolyte and a sustainable organic cathode. J Power Sources 399:294–298. https://doi.org/10.1016/j.jpowsour.2018.07.124
Fei H et al (2019) Safe all-solid-state potassium batteries with three dimentional, flexible and binder-free metal sulfide array electrode. J Power Sources 433:226697. https://doi.org/10.1016/J.JPOWSOUR.2019.226697
Shi J, Vincent CA (1993) The effect of molecular weight on cation mobility in polymer electrolytes. Solid State Ionics 60(1–3):11–17. https://doi.org/10.1016/0167-2738(93)90268-8
Vincent CA (1995) Ion transport in polymer electrolytes. Electrochim Acta 40(13–14):2035–2040. https://doi.org/10.1016/0013-4686(95)00138-5
Acosta JL, Morales E (1998) Synthesis and characterization of polymeric electrolytes for solid state magnesium batteries. Electrochim Acta 43(7):791–797. https://doi.org/10.1016/S0013-4686(97)00123-0
Anilkumar KM, Jinisha B, Manoj M, Jayalekshmi S (2017) Poly(ethylene oxide) (PEO) – poly(vinyl pyrrolidone) (PVP) blend polymer based solid electrolyte membranes for developing solid state magnesium ion cells. Eur Polym J 89:249–262. https://doi.org/10.1016/J.EURPOLYMJ.2017.02.004
Thelen JL, Inceoglu S, Venkatesan NR, Mackay NG, Balsara NP (2016) Relationship between ion dissociation, melt morphology, and electrochemical performance of lithium and magnesium single-ion conducting block copolymers. Macromolecules 49(23):9139–9147. https://doi.org/10.1021/ACS.MACROMOL.6B01886/SUPPL_FILE/MA6B01886_SI_001.PDF
Park B, Schaefer JL (2020) Review—Polymer electrolytes for magnesium batteries: forging away from analogs of lithium polymer electrolytes and towards the rechargeable magnesium metal polymer battery. J Electrochem Soc 167(7):070545. https://doi.org/10.1149/1945-7111/AB7C71
Agrawal RC, Sahu DK, Mahipal YK, Ashrafi R (2013) Investigations on ion transport properties of hot-press cast magnesium ion conducting nano-composite polymer electrolyte (NCPE) films: effect of filler particle dispersal on room temperature conductivity. Mater Chem Phys 139(2–3):410–415. https://doi.org/10.1016/J.MATCHEMPHYS.2012.12.056
Peled E (1979) The electrochemical behavior of alkali and alkaline earth metals in nonaqueous battery systems—the solid electrolyte interphase model. J Electrochem Soc 126(12):2047–2051. https://doi.org/10.1149/1.2128859
Peled E, Golodnitsky D, Penciner J (2007) The anode/electrolyte interface. Handb Batter Mater 419–456. https://doi.org/10.1002/9783527611676.CH18
Lee B, Paek E, Mitlin D, Lee SW (2019) Sodium metal anodes: emerging solutions to dendrite growth. Chem Rev 119(8). https://doi.org/10.1021/ACS.CHEMREV.8B00642
Fincher CD, Zhang Y, Pharr GM, Pharr M (2020) Elastic and plastic characteristics of sodium metal. ACS Appl Energy Mater 3(2):1759–1767. https://doi.org/10.1021/ACSAEM.9B02225
Zhang W, De Zhao C, Wu XL (2020) Research progresses on interfaces in solid-state sodium batteries: a topic review. Adv Mater Interfaces 7(23):2001444. https://doi.org/10.1002/ADMI.202001444
Duchêne L et al (2017) A stable 3 V all-solid-state sodium–ion battery based on a closo-borate electrolyte. Energy Environ Sci 10(12):2609–2615. https://doi.org/10.1039/C7EE02420G
Jo JH et al (2018) Sodium-ion batteries: building effective layered cathode materials with long-term cycling by modifying the surface via sodium phosphate. Adv Funct Mater 28(14):1705968. https://doi.org/10.1002/ADFM.201705968
Yue J et al (2018) Long cycle life all-solid-state sodium ion battery. ACS Appl Mater Interf 10(46):39645–39650. https://doi.org/10.1021/ACSAMI.8B12610
Banerjee A et al (2016) Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew Chemie Int Ed 55(33):9634–9638. https://doi.org/10.1002/ANIE.201604158
Yamauchi H, Ikejiri J, Sato F, Oshita H, Honma T, Komatsu T (2019) Pressureless all-solid-state sodium-ion battery consisting of sodium iron pyrophosphate glass-ceramic cathode and β″-alumina solid electrolyte composite. J Am Ceram Soc 102(11):6658–6667. https://doi.org/10.1111/JACE.16607
Yamauchi H et al (2020) Enhanced rate capabilities in a glass-ceramic-derived sodium all-solid-state battery. Sci Rep 10(1):1–12. https://doi.org/10.1038/s41598-020-66410-1
Hou W, Guo X, Shen X, Amine K, Yu H, Lu J (2018) Solid electrolytes and interfaces in all-solid-state sodium batteries: progress and perspective. Nano Energy 52:279–291. https://doi.org/10.1016/J.NANOEN.2018.07.036
Seh ZW, Sun J, Sun Y, Cui Y (2015) A highly reversible room-temperature sodium metal anode. ACS Cent Sci 1(8):449–455. https://doi.org/10.1021/ACSCENTSCI.5B00328
Cohn AP, Muralidharan N, Carter R, Share K, Pint CL (2017) Anode-free sodium battery through in situ plating of sodium metal. Nano Lett 17(2):1296–1301. https://doi.org/10.1021/ACS.NANOLETT.6B05174
Lee SE, Tang MH (2019) Reliable reference electrodes for nonaqueous sodium-ion batteries. J Electrochem Soc 166(14):3260–3264. https://doi.org/10.1149/2.0401914jes
Oh JAS, He L, Chua B, Zeng K, Lu L (2021) Inorganic sodium solid-state electrolyte and interface with sodium metal for room-temperature metal solid-state batteries. Energy Storage Mater 34:28–44. https://doi.org/10.1016/J.ENSM.2020.08.037
Lu X et al (2014) Liquid-metal electrode to enable ultra-low temperature sodium–beta alumina batteries for renewable energy storage. Nat Commun 5(1):1–8. https://doi.org/10.1038/ncomms5578
Baclig AC, McConohy G, Poletayev A, Lee J-H, Chueh WC, Rugolo J (2018) High-voltage, room-temperature liquid metal flow battery enabled by Na-K | K-β″-alumina stability. Joule 2:1287–1296. https://doi.org/10.1016/j.joule.2018.04.008
Bommier C, Ji X, Greaney PA (2018) Electrochemical properties and theoretical capacity for sodium storage in hard carbon: insights from first principles calculations. https://doi.org/10.1021/acs.chemmater.8b01390
Hou H, Qiu X, Wei W, Zhang Y, Ji X (2017) Carbon anode materials for advanced sodium-ion batteries. Adv Energy Mater 7(24):1602898. https://doi.org/10.1002/AENM.201602898
Perveen T, Siddiq M, Shahzad N, Ihsan R, Ahmad A, Shahzad MI (2020) Prospects in anode materials for sodium ion batteries - a review. Renew Sustain Energy Rev 119:109549. https://doi.org/10.1016/J.RSER.2019.109549
Stevens DA, Dahn JR (2000) High capacity anode materials for rechargeable sodium-ion batteries. J Electrochem Soc 147(4):1271. https://doi.org/10.1149/1.1393348/XML
Yang J et al (2020) Guided-formation of a favorable interface for stabilizing Na metal solid-state batteries. J Mater Chem A 8(16):7828–7835. https://doi.org/10.1039/D0TA01498B
Rao RP, Zhang X, Phuah KC, Adams S (2019) Mechanochemical synthesis of fast sodium ion conductor Na11Sn2PSe12 enables first sodium–selenium all-solid-state battery. J Mater Chem A 7(36):20790–20798. https://doi.org/10.1039/C9TA06279C
Xiang X, Zhang K, Chen J (2015) Recent advances and prospects of cathode materials for sodium-ion batteries. Adv Mater 27(36):5343–5364. https://doi.org/10.1002/ADMA.201501527
Islam MS, Fisher CAJ (2014) Lithium and sodium battery cathode materials: computational insights into voltage, diffusion and nanostructural properties. Chem Soc Rev 43(1):185–204. https://doi.org/10.1039/C3CS60199D
Wang L, Chen B, Ma J, Cui G, Chen L (2018) Reviving lithium cobalt oxide-based lithium secondary batteries-toward a higher energy density. Chem Soc Rev 47(17):6505–6602. https://doi.org/10.1039/C8CS00322J
Dai Z et al (2017) Advanced cathode materials for sodium-ion batteries: what determines our choices? Small Methods 1(5):1700098. https://doi.org/10.1002/SMTD.201700098
Qian J et al (2018) Prussian blue cathode materials for sodium-ion batteries and other ion batteries. Adv Energy Mater 8(17):1702619. https://doi.org/10.1002/AENM.201702619
Kim D et al (2011) Enabling sodium batteries using lithium-substituted sodium layered transition metal oxide cathodes. Adv Energy Mater 1(3):333–336. https://doi.org/10.1002/AENM.201000061
Wu H, Zou X, Wu XL (2020) Nanoconstruction and nanoeffect of phosphate-based cathode materials for advanced sodium-ion batteries. Nano Futur 4(4):042001. https://doi.org/10.1088/2399-1984/ABC103
Gu ZY et al (2020) Carbon-coating-increased working voltage and energy density towards an advanced Na3V2(PO4)2F3@C cathode in sodium-ion batteries. Sci Bull 65(9):702–710. https://doi.org/10.1016/J.SCIB.2020.01.018
Serras P et al (2012) High voltage cathode materials for Na-ion batteries of general formula Na3V2O2x(PO4)2F3−2x. J Mater Chem 22(41):22301–22308. https://doi.org/10.1039/C2JM35293A
Lee HW, Wang RY, Pasta M, Lee SW, Liu N, Cui Y (2014) Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat Commun 5. https://doi.org/10.1038/NCOMMS6280
Zheng X, Bommier C, Luo W, Jiang L, Hao Y, Huang Y (2019) Sodium metal anodes for room-temperature sodium-ion batteries: applications, challenges and solutions. Energy Storage Mater 16:6–23. https://doi.org/10.1016/J.ENSM.2018.04.014
Zheng Y et al (2018) High-capacity all-solid-state sodium metal battery with hybrid polymer electrolytes. Adv Energy Mater 8(27):1801885. https://doi.org/10.1002/AENM.201801885
Song S, Duong HM, Korsunsky AM, Hu N, Lu L (2016) A Na(+) superionic conductor for room-temperature sodium batteries. Sci Rep 6. https://doi.org/10.1038/SREP32330
Yang J et al (2020) Ultrastable all-solid-state sodium rechargeable batteries. ACS Energy Lett 5(9):2835–2841. https://doi.org/10.1021/ACSENERGYLETT.0C01432/SUPPL_FILE/NZ0C01432_SI_001.PDF
Yao Y et al (2020) Toward high energy density all solid-state sodium batteries with excellent flexibility. Adv Energy Mater 10(12):1903698. https://doi.org/10.1002/AENM.201903698
Ruan Y, Guo F, Liu J, Song S, Jiang N, Cheng B (2019) Optimization of Na3Zr2Si2PO12 ceramic electrolyte and interface for high performance solid-state sodium battery. Ceram Int 45(2):1770–1776. https://doi.org/10.1016/J.CERAMINT.2018.10.062
Wang Z et al (2020) Tuning rate-limiting factors to achieve ultrahigh-rate solid-state sodium-ion batteries. ACS Appl Mater Interfaces 12(43):48677–48683. https://doi.org/10.1021/ACSAMI.0C15015
Liu S et al (2021) Manipulating the solvation structure of nonflammable electrolyte and interface to enable unprecedented stability of graphite anodes beyond 2 years for safe potassium-ion batteries. Adv Mater 33(1):2006313. https://doi.org/10.1002/ADMA.202006313
Zhang Q et al (2021) Enabling atomic-scale imaging of sensitive potassium metal and related solid electrolyte interphases using ultralow-dose cryo-TEM. Adv Mater 33(43). https://doi.org/10.1002/ADMA.202102666/FORMAT/PDF
Liu S et al (2020) An intrinsically non-flammable electrolyte for high-performance potassium batteries. Angew Chemie 132(9):3667–3673. https://doi.org/10.1002/ANGE.201913174
Shi P et al (2021) Red phosphorous-derived protective layers with high ionic conductivity and mechanical strength on dendrite-free sodium and potassium metal anodes. Adv Energy Mater 11(5):2003381. https://doi.org/10.1002/AENM.202003381
Xiao N, Zheng J, Gourdin G, Schkeryantz L, Wu Y (2019) Anchoring an artificial protective layer to stabilize potassium metal anode in rechargeable K-O2 batteries. ACS Appl Mater Interfaces 11(18):16571–16577. https://doi.org/10.1021/ACSAMI.9B02116/SUPPL_FILE/AM9B02116_SI_001.PDF
Lu K, Zhang H, Gao S, Cheng Y, Ma H (2018) High rate and stable symmetric potassium ion batteries fabricated with flexible electrodes and solid-state electrolytes. Nanoscale 10(44):20754–20760. https://doi.org/10.1039/C8NR07268J
Chen Y et al (2015) Organic electrode for non-aqueous potassium-ion batteries. Nano Energy 18:205–211. https://doi.org/10.1016/j.nanoen.2015.10.015
Bella F et al (2021) An overview on anodes for magnesium batteries: challenges towards a promising storage solution for renewables. Nanomater 11(3):810. https://doi.org/10.3390/NANO11030810
Di Leo RA, Zhang Q, Marschilok AC, Takeuchi KJ, Takeuchi ES (2015) Composite anodes for secondary magnesium ion batteries prepared via electrodeposition of nanostructured bismuth on carbon nanotube substrates. ECS Electrochem Lett 4(1):A10–A14. https://doi.org/10.1149/2.0081501EEL/XML
Yaghoobnejad Asl H, Fu J, Kumar H, Welborn SS, Shenoy VB, Detsi E (2018) In situ dealloying of bulk Mg2Sn in Mg-ion half cell as an effective route to nanostructured Sn for high performance Mg-ion battery anodes. Chem Mater 30(5):1815–1824. https://doi.org/10.1021/ACS.CHEMMATER.7B04124/SUPPL_FILE/CM7B04124_SI_001.PDF
Niu J et al (2018) Dual phase enhanced superior electrochemical performance of nanoporous bismuth-tin alloy anodes for magnesium-ion batteries. Energy Storage Mater 14:351–360. https://doi.org/10.1016/J.ENSM.2018.05.023
Imhof R, Haas O, Novák P (1999) Magnesium insertion electrodes for rechargeable nonaqueous batteries—a competitive alternative to lithium? Electrochim Acta 45
Aurbach D et al (2000) Prototype systems for rechargeable magnesium batteries. Nature 407:724–727. https://doi.org/10.1002/chin.200103014
Aurbach D et al (2007) Progress in rechargeable magnesium battery technology. Adv Mater 19(23):4260–4267. https://doi.org/10.1002/adma.200701495
Gautam GS, Canepa P, Malik R, Liu M (2015) First-principles evaluation of multi-valent cation insertion into orthorhombic V2O5. ChemComm 51:13619–13622. https://doi.org/10.1039/c5cc04947d
Gautam GS, Canepa P, Richards WD, Malik R, Ceder G (2016) Role of structural H2O in intercalation electrodes: the case of Mg in nanocrystalline xerogel - V2O5. Nano Lett 16:2426–2431. https://doi.org/10.1021/acs.nanolett.5b05273
Gregory TD (1990) Nonaqueous electrochemistry of magnesium. J Electrochem Soc 137(3):775. https://doi.org/10.1149/1.2086553
Sai Gautam G, Canepa P, Abdellahi A, Urban A, Malik R, Ceder G (2015) The intercalation phase diagram of Mg in V2O5 from first-principles. Chem Mater 27(10):3733–3742. https://doi.org/10.1021/acs.chemmater.5b00957
Mukherjee A, Sa N, Phillips PJ, Burrell A, Vaughey J, Klie RF (2017) Direct investigation of Mg intercalation into the orthorhombic V2O5 cathode using atomic-resolution transmission electron microscopy. Chem Mater 29(5):2218–2226. https://doi.org/10.1021/acs.chemmater.6b05089
Gershinsky G, Yoo HD, Gofer Y, Aurbach D (2013) Electrochemical and spectroscopic analysis of Mg2+ intercalation into thin film electrodes of layered oxides: V2O5 and MoO3. Langmuir 29(34):10964–10972. https://doi.org/10.1021/la402391f
Fu Q et al (2019) In operando synchrotron diffraction and in operando X-ray absorption spectroscopy investigations of orthorhombic V2O5 nanowires as cathode materials for Mg-ion batteries. J Am Chem Soc 141(6):2305–2315. https://doi.org/10.1021/jacs.8b08998
Tang PE, Sakamoto JS, Baudrin E, Dunn B (2004) V2O5 aerogel as a versatile host for metal ions. J Non Cryst Solids 350:67–72. https://doi.org/10.1016/j.jnoncrysol.2004.07.072
Novák P (1993) Electrochemical insertion of magnesium in metal oxides and sulfides from aprotic electrolytes. J Electrochem Soc 140(1):140. https://doi.org/10.1149/1.2056075
Yu L, Zhang X (2004) Electrochemical insertion of magnesium ions into V2O5 from aprotic electrolytes with varied water content. J Colloid Interface Sci 278(1):160–165. https://doi.org/10.1016/j.jcis.2004.05.028
Kim RH et al (2014) Highly reduced VOx nanotube cathode materials with ultra-high capacity for magnesium ion batteries. J Mater Chem A 2(48):20636–20641. https://doi.org/10.1039/c4ta05564k
Cheng Y et al (2016) Molecular storage of Mg ions with vanadium oxide nanoclusters. Adv Funct Mater 26(20):3446–3453. https://doi.org/10.1002/adfm.201505501
Andrews JL et al (2018) Reversible Mg-ion insertion in a metastable one-dimensional polymorph of V2O5. Chem 4(3):564–585. https://doi.org/10.1016/j.chempr.2017.12.018
Sa N et al (2016) Is alpha-V2O5 a cathode material for Mg insertion batteries? J Power Sources 323:44–50. https://doi.org/10.1016/j.jpowsour.2016.05.028
Mukherjee A, Taragin S, Aviv H, Perelshtein I, Noked M (2020) Rationally designed vanadium pentoxide as high capacity insertion material for Mg-ion. Adv Funct Mater 30(38):1–9. https://doi.org/10.1002/adfm.202003518
Zhao-Karger Z, Zhao X, Wang D, Diemant T, Behm RJ, Fichtner M (2015) Performance improvement of magnesium sulfur batteries with modified non-nucleophilic electrolytes. Adv Energy Mater 5. https://doi.org/10.1002/aenm.201401155
Kim N, Jinks-Robertson S (2012) Transcription as a source of genome instability. Nat Rev Genet 13(3):204–214. https://doi.org/10.1038/nrg3152
Gao T et al (2015) Enhancing the reversibility of Mg/S battery chemistry through Li+ mediation. J Am Chem Soc. https://doi.org/10.1021/jacs.5b07820
Bitenc J et al (2015) Anthraquinone-based polymer as cathode in rechargeable magnesium batteries. Chemsuschem 8(24):4128–4132. https://doi.org/10.1002/cssc.201500910
Abouzari-Lotf E et al (2021) A self-conditioned metalloporphyrin as a highly stable cathode for fast rechargeable magnesium batteries. Chemsuschem 14(8):1840–1846. https://doi.org/10.1002/cssc.202100340
Du A et al (2019) A crosslinked polytetrahydrofuran-borate-based polymer electrolyte enabling wide-working-temperature-range rechargeable magnesium batteries. Adv Mater 31(11):1805930. https://doi.org/10.1002/ADMA.201805930
Author information
Authors and Affiliations
Corresponding author
Additional information
For the special issue dedicated to the 70th birthday of Doron Aurbach.
This manuscript is dedicated to Professor Doron Aurbach, honoring his platinum jubilee. Professor Aurbach served as a faculty member of the Chemistry Department at Bar-Ilan University with distinction for more than thirty years. Prof. Aurbach educated few generations of materials scientists and electrochemists from all over the world, and his students are now educating others in various academic institutions and industrial entities. Doron, as a teacher and as a mentor repeatedly claimed: “I have learned from all my teachers, and I've learned even more from my students”. Therefore, debating and discussing with students, colleagues and collaborators were always part of the scientific culture in his lab. Thank you Doron, for being a teacher, a colleague and a friend. The authors wish you many more years of scientific adventures.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
York, M., Larson, K., Harris, K.C. et al. Recent advances in solid-state beyond lithium batteries. J Solid State Electrochem 26, 1851–1869 (2022). https://doi.org/10.1007/s10008-022-05223-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05223-w