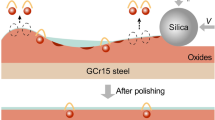
Abstract
Excellent surface quality is strongly needed for improving the service performance of bearings under severe lubrication conditions. In this study, chemical mechanical polishing (CMP) was used to process GCr15 bearing steel. Oxalic acid and H2O2 were used as critical additives in the CMP slurries. In the presence of oxalic acid and with the increasing H2O2, the material removal rate (MRR) first increases sharply, then gradually decreases and reaches a plateau, and then decreases again. In particular, with the addition of 0.1 M oxalic acid and 1.5 wt% H2O2, a satisfactory CMP performance can be realized. The MRR is as high as 462 nm/min, and the surface roughness Ra is as low as 2.1 nm. During the CMP process, the surface film is composed of insoluble Fe3+ oxides and Fe-oxalic acid compounds. Insoluble Fe3+ oxides and FeC2O4 can effectively suppress the corrosion, leading to the low surface roughness. The formation of Fe-oxalic acid compounds, especially soluble ones, may weaken the surface film, resulting in the high MRR. A one-step CMP method was developed. Within 14 min, the Ra of GCr15 steel decreases from 249.3 nm to about 2.0 nm. This study provides a promising CMP method for bearing steel.











Similar content being viewed by others
References
Yin F, Hua L, Mao H, Han X (2013) Constitutive modeling for flow behavior of GCr15 steel under hot compression experiments. Mater Design 43:393–401. https://doi.org/10.1016/j.matdes.2012.07.009
Beswick JM (ed) (2002) Bearing steel technology. ASTM International, Philadelphia, PA
Ueda T, Mitamura N (2009) Mechanism of dent initiated flaking and bearing life enhancement technology under contaminated lubrication condition. Part II: Effect of rolling element surface roughness on flaking resulting from dents, and life enhancement technology of rolling bearings under contaminated lubrication condition. Tribol Int 42(11):1832–1837. https://doi.org/10.1016/j.triboint.2008.12.010
Sedlaček M, Podgornik B, Vižintin J (2009) Influence of surface preparation on roughness parameters, friction and wear. Wear 266(3):482–487. https://doi.org/10.1016/j.wear.2008.04.017
Kao MJ, Hsu FC, Peng DX (2014) Synthesis and characterization of SiO2 nanoparticles and their efficacy in chemical mechanical polishing steel substrate. Adv Mater Sci Eng 2014:1–8. https://doi.org/10.1155/2014/691967
Peng D-X (2014) Optimization of chemical mechanical polishing parameters on surface roughness of steel substrate with aluminum nanoparticles via Taguchi approach. Ind Lubr Tribol 66(6):685–690. https://doi.org/10.1108/ilt-07-2012-0063
Peng D-X (2014) Chemical mechanical polishing of steel substrate using aluminum nanoparticles abrasive slurry. Ind Lubr Tribol 66(1):124–130. https://doi.org/10.1108/ilt-10-2011-0078
Jiang L, He Y, Luo J (2015) Chemical mechanical polishing of steel substrate using colloidal silica-based slurries. Appl Surf Sci 330:487–495. https://doi.org/10.1016/j.apsusc.2015.01.016
Wu H, Jiang L, Liu J, Deng C, Huang H, Qian L (2020) Efficient chemical mechanical polishing of AISI 52100 bearing steel with TiSol-NH4 dispersion-based slurries. Tribol Lett 68(1):34. https://doi.org/10.1007/s11249-020-1274-4
Liu J, Jiang L, Wu H, Zhong X, Qian L (2021) Performance of carboxyl groups in chemical mechanical polishing of GCr15 bearing steel: Effects of carbon chain length and pH. Tribol Lett 69(4):161. https://doi.org/10.1007/s11249-021-01532-9
Li J, Liu Y, Wang T, Lu X, Luo J (2013) Electrochemical investigation of copper passivation kinetics and its application to low-pressure CMP modeling. Appl Surf Sci 265:764–770. https://doi.org/10.1016/j.apsusc.2012.11.106
Liu J, Jiang L, Wu H, Zhao T, Qian L (2020) 5-Methyl-1H-Benzotriazole as an effective corrosion inhibitor for ultra-precision chemical mechanical polishing of bearing steel. J Electrochem Soc 167(13):131502. https://doi.org/10.1149/1945-7111/abb0d9
Wu H, Jiang L, Zhong X, Liu J, Qin N, Qian L (2020) Exploring the role of −NH2 functional groups of ethylenediamine in chemical mechanical polishing of GCr15 bearing steel. Friction. https://doi.org/10.1007/s40544-020-0460-6
Lee SO, Tran T, Jung BH, Kim SJ, Kim MJ (2007) Dissolution of iron oxide using oxalic acid. Hydrometallurgy 87(3):91–99. https://doi.org/10.1016/j.hydromet.2007.02.005
Borghi EB, Alí SP, Morando PJ, Blesa MA (1996) Cleaning of stainless steel surfaces and oxide dissolution by malonic and oxalic acids. J Nucl Mater 229:115–123. https://doi.org/10.1016/0022-3115(95)00201-4
Gorantla VRK, Babel A, Pandija S, Babu SV (2005) Oxalic acid as a complexing agent in CMP slurries for copper. Electrochem Solid-State Lett 8(5):G131–G134. https://doi.org/10.1149/1.1883873
Li Y (2007) Microelectronic applications of chemical mechanical planarization. John Wiley & Sons, Inc., Hoboken, New Jersey, USA. https://doi.org/10.1002/9780470180907
Zhao D, Lu X (2013) Chemical mechanical polishing: Theory and experiment. Friction 1(4):306–326. https://doi.org/10.1007/s40544-013-0035-x
Stahl PH, Wermuth CG (2008) Handbook of pharmaceutical salts properties, selection, and use. Wiley-VCH
Locke MJ, McIver RT (1983) Effect of solvation on the acid/base properties of glycine. J Am Chem Soc 105(13):4226–4232. https://doi.org/10.1021/ja00351a017
University W (2016) Analytical Chemistry (in Chinese). Higher Education Press, Beijing
Panias D, Taxiarchou M, Paspaliaris I, Kontopoulos A (1996) Mechanisms of dissolution of iron oxides in aqueous oxalic acid solutions. Hydrometallurgy 42(2):257–265. https://doi.org/10.1016/0304-386X(95)00104-O
Kim YJ, Kwon OJ, Kang MC, Kim JJ (2011) Effects of the functional groups of complexing agents and Cu oxide formation on Cu dissolution behaviors in Cu CMP process. J Electrochem Soc 158(2):H190–H196. https://doi.org/10.1149/1.3522811
Martell AE, Motekaitis RJ, Chen D, Hancock RD, McManus D (1996) Selection of new Fe(III)/Fe(II) chelating agents as catalysts for the oxidation of hydrogen sulfide to sulfur by air. Can J Chem 74(10):1872–1879. https://doi.org/10.1139/v96-210
Wu H, Jiang L, Zhong X, Liu J, Qin N, Qian L (2021) Exploring the role of −NH2 functional groups of ethylenediamine in chemical mechanical polishing of GCr15 bearing steel. Friction 9(6):1673–1687. https://doi.org/10.1007/s40544-020-0460-6
Biesinger MC, Payne BP, Grosvenor AP, Lau LWM, Gerson AR, Smart RSC (2011) Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl Surf Sci 257(7):2717–2730. https://doi.org/10.1016/j.apsusc.2010.10.051
Keller P, Strehblow H-H (2004) XPS investigations of electrochemically formed passive layers on Fe/Cr-alloys in 0.5 M H2SO4. Corros Sci 46(8):1939–1952. https://doi.org/10.1016/j.corsci.2004.01.007
Lin T-C, Seshadri G, Kelber JA (1997) A consistent method for quantitative XPS peak analysis of thin oxide films on clean polycrystalline iron surfaces. Appl Surf Sci 119(1):83–92. https://doi.org/10.1016/S0169-4332(97)00167-0
Taheri P, Wielant J, Hauffman T, Flores JR, Hannour F, de Wit JHW, Mol JMC, Terryn H (2011) A comparison of the interfacial bonding properties of carboxylic acid functional groups on zinc and iron substrates. Electrochim Acta 56(4):1904–1911. https://doi.org/10.1016/j.electacta.2010.10.079
Huang J, Wu X, Han E-H (2009) Influence of pH on electrochemical properties of passive films formed on Alloy 690 in high temperature aqueous environments. Corros Sci 51(12):2976–2982. https://doi.org/10.1016/j.corsci.2009.08.002
Lee W-J (2003) Inhibiting effects of imidazole on copper corrosion in 1 M HNO3 solution. Mater Sci Eng, A 348(1):217–226. https://doi.org/10.1016/S0921-5093(02)00734-7
Amin MA, Abd El-Rehim SS, El-Sherbini EEF, Bayoumi RS (2007) The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid: Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim Acta 52(11):3588–3600. https://doi.org/10.1016/j.electacta.2006.10.019
Cao C (2008) Principles of electrochemistry of corrosion (in Chinese). Chemistry and Industry Press
Bordbar-Khiabani A, Ebrahimi S, Yarmand B (2019) Highly corrosion protection properties of plasma electrolytic oxidized titanium using rGO nanosheets. Appl Surf Sci 486:153–165. https://doi.org/10.1016/j.apsusc.2019.05.026
Sekine I, Okano C, Yuasa M (1990) The corrosion behaviour of ferritic stainless steel in oxalic acid solutions. Corros Sci 30(4):351–366. https://doi.org/10.1016/0010-938X(90)90043-5
Beverskog B, Puigdomenech I (1996) Revised pourbaix diagrams for iron at 25–300 °C. Corros Sci 38(12):2121–2135. https://doi.org/10.1016/S0010-938X(96)00067-4
Dong J, Dong J, Han E, Liu C, Ke W (2009) Rusting evolvement of mild steel under wet/dry cyclic condition with pH 4.00 NaHSO3 solution. Corros Sci Prot Technol (in Chinese) 21(1):1–4
Dong J, Wei K (2009) The accelerated test of simulated atmospheric corrosion and the rust evolution of low carbon steel. Electrochemistry (in Chinese) 15(02):170–178
Xu L, Wang J (2011) A heterogeneous Fenton-like system with nanoparticulate zero-valent iron for removal of 4-chloro-3-methyl phenol. J Hazard Mater 186(1):256–264. https://doi.org/10.1016/j.jhazmat.2010.10.116
Kang YW, Hwang K-Y (2000) Effects of reaction conditions on the oxidation efficiency in the Fenton process. Water Res 34(10):2786–2790. https://doi.org/10.1016/S0043-1354(99)00388-7
Imlay J, Chin S, Linn S (1988) Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240(4852):640–642. https://doi.org/10.1126/science.2834821
Du CW, Li XG, Liang P, Liu ZY, Jia GF, Cheng YF (2009) Effects of Microstructure on Corrosion of X70 Pipe Steel in an Alkaline Soil. J Mater Eng Perform 18(2):216–220. https://doi.org/10.1007/s11665-008-9280-y
Tripathi S, Choi S, Doyle FM, Dornfeld DA (2009) Integrated tribo-chemical modeling of copper CMP. In: MRS Spring Meeting, San Francisco, April 14–16, 2009. eScholarship University of California
Choi S, Tripathi S, Dornfeld DA, Doyle FM (2010) Copper CMP modeling: Millisecond scale adsorption kinetics of BTA in glycine-containing solutions at pH 4. J Electrochem Soc 157(12):H1153–H1159. https://doi.org/10.1149/1.3499217
Ramakrishnan S, Janjam SVSB, Patri UB, Roy D, Babu SV (2007) Comparison of dicarboxylic acids as complexing agents for abrasive-free chemical mechanical planarization of copper. Microelectron Eng 84(1):80–86. https://doi.org/10.1016/j.mee.2006.08.011
Balmer ME, Sulzberger B (1999) Atrazine degradation in irradiated iron/oxalate systems: Effects of pH and oxalate. Environ Sci Technol 33(14):2418–2424. https://doi.org/10.1021/es9808705
Zhang Z, Liu W, Song Z, Hu X (2010) Two-step chemical mechanical polishing of sapphire substrate. J Electrochem Soc 157(6):H688. https://doi.org/10.1149/1.3410116
Jiang L, He Y, Yang Y, Luo J (2015) Chemical mechanical polishing of stainless steel as solar cell substrate. ECS J Solid State Sci Technol 4(5):P162–P170. https://doi.org/10.1149/2.0171505jss
Wang C, Gao J, Tian J, Niu X, Liu Y (2013) Chemical mechanical planarization of barrier layers by using a weakly alkaline slurry. Microelectron Eng 108:71–75. https://doi.org/10.1016/j.mee.2013.04.001
Acknowledgements
The authors are grateful for the financial support by the National Key R&D Program of China (2020YFA0711001), National Natural Science Foundation of China (51975488 and 51991373), National Key R&D Program of China (2018YFB2000400), Fundamental Research Funds for the Central Universities (2682021CG011), and Beijing Key Laboratory of Long-life Technology of Precise Rotation and Transmission Mechanisms (BZ0388201902).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, J., Jiang, L., Xiao, G. et al. High-performance chemical mechanical polishing of GCr15 bearing steel enabled by the synergistic action of oxalic acid and H2O2. J Solid State Electrochem 26, 809–820 (2022). https://doi.org/10.1007/s10008-022-05122-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-022-05122-0