Abstract
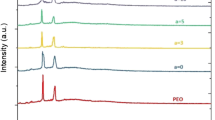
Flexible and free-standing electrolyte membranes of nanocomposite ‘poly(ethylene oxide) (PEO)/starch-nanocrystals (SNCs)’ complexed with magnesium bromide (MgBr2) salt at various concentrations (5, 10, 15, 20, and 25 WT.%) were prepared using conventional solution casting technique. The microstructural and thermal stability properties of the pure and MgBr2 salt complexed PEO/SNC nanocomposite membranes were characterized by scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared (FTIR) spectroscopy, and differential scanning calorimetry (DSC). Complex electrochemical impedance spectroscopy (EIS) and dielectric studies of the nanocomposite membranes were carried out over the frequency range 0.1–1 MHz and within the temperature range of 30–70 °C. Concerning pure PEO/SNCs (10 WT.%), the electrolyte membrane of the composition ‘PEO/SNCs (10 WT.%)/MgBr2 (25 WT.%)’ demonstrated more than three orders of magnitude in the room temperature ionic conductivity, as measured by EIS. A clear shift in the position of the dielectric relaxation peaks was noticed as a function of salt doping concentration in \(\partial log\left({\varepsilon }^{{^{\prime}}}\right)/\partial log\left(\omega \right) versus log\left(\omega \right)\) spectra. It was estimated by dielectric spectroscopy that the values of the diffusion coefficient (D) and the total ion concentration (n) for the studied nanocomposite electrolyte membranes were increased in proportion to the doping salt concentrations.





















Similar content being viewed by others
References
Ge S, Leng Y, Liu T, Longchamps RS, Yang XG, Gao Y, Wang D, Wang CY (2020) Sci Adv 6:1–8
Yamada Y, Wang J, Ko S, Watanabe E, Yamada A (2019) Nat Energy 4:269–280
Deng J, Bae C, Marcicki J, Masias A, Miller T (2018) Nat Energy 3:261–266
Du A, Zhang H, Zhang Z, Zhao J, Cui Z, Zhao Y, Dong S, Wang L, Zhou X, Cui G (2019) Adv Mater 31:1805930
Yao P, Yu H, Ding Z, Liu Y, Lu J, Lavorgna M, Wu J, Liu X (2019) Front Chem 7:1–17
Zhang Q, Liu K, Ding F, Liu X (2017) Nano Res 10:4139–4174
Xue Z, He D, Xie X (2015) J Mater Chem A 3:19218–19253
Xiao Z, Zhou B, Wang J, Zuo C, He D, Xie X, Xue Z (2019) J Membr Sci 576:182–189
Zhou B, He D, Hu J, Ye Y, Peng H, Zhou X, Xie X, Xue Z (2018) J Mater Chem A 6:11725–11733
Paulkner RDA, Kulkarni AR, Jonscher A (1983) Dielectric relaxation in solids, dielectric (Eds.), Solid State Ionics – Materials and Applications, Press, London
Bouridah A, Dalard F, Deroo D, Cheradame H, Le JF (1985) Solid State Ion 15:233–240
Scrosati B (1993) Application of electroactive polymers. Chapman and Hall, London
Weston JE, Steele BCH (1982) Solid State Ion 7:75–79
Benrabah D, Sanchez JY, Armand M (1992) Electrochim Acta 37:1737–1741
Giannelis EP (1996) Adv Mater 8:29–35
Liu F, Hu N, Zhang J, Atobe S, Weng S, Ning H, Liu Y, Wu L, Zhao Y, Mo F, Fu S, Xu C (2016) RSC Adv 6:66658–66664
Lipatov YS (1995) Polymer reinforcement. Chem Tec Publishing, Ukraine
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nature 394:456–458
Florjanczyk Z, Marcinek M, Wieczorek W, Langwald N (2004) Pol J Chem 78:1279–1304
Gross RA, Kalra B (2002) Science 297:803–807
Domene-López D, Guillén M, Martin-Gullon I, García-Quesada JC, Montalbán MG (2018) Carbohydr Polym 202:299–305
Souza AC, Benze R, Ferrão ES, Ditchfield C, Coelho ACV, Tadini CC (2012) LWT - Food Sci Technol 46:110–117
Abdelghany AM, Oraby AH, Asnag GM (2019) J Mol Struct 1180:15–25
Ramly K, Khiar ASA (2015) Appl Mech Mater 754–755:29–33
Yu F, Prashantha K, Soulestin J, Lacrampe MF, Krawczak P (2013) Carbohydr Polym 91:253–261
Jagadish RS, Raj B, Parameswara P, Somashekar R (2013) J Appl Polym Sci 127:1191–1197
Pereira AGB, Gouveia RF, de Carvalho GM, Rubira AF, Muniz EC (2009) Mater Sci Eng C 29:499–504
Angellier H, Molina-Boisseau S, Dufresne A (2006) Macromol Symp 233:132–136
Battista OA (1975) Microcrystal polymer science. McGraw-Hill Book Company, New York
Hassoun J, Lee KS, Sun YK, Scrosati B (2011) J Am Chem Soc 133:3139–3143
Vignarooban K, Kushagra R, Elango A, Badami P, Mellander B -E, Xu X, Tucker TG, Nam C, Kannan AM (2016) Int J Hydrog Energy 41: 2829 – 2846
Song J, Sahadeo E, Noked M, Lee SB (2016) J Phys Chem Lett 7:1736–1749
Muldoon J, Bucur CB, Gregory T (2017) Angew Chem Int Ed 56:12064–12084
Eslam MS, Mona MN, El-Mansy MK (2015) J Adv Res 6:563–569
Emmanuel O, Ewomazino O, Tizazu M (2018) Eur Polym J 108:570–581
Angellier H, Choisnard L, Molina-Boisseau S, Ozil P, Dufresne A (2004) Biomacromol 5:1545–1551
Xi J, Qiu X, Cui M, Tang X, Zhu W, Chen L (2006) J Power Sources 156:581–588
Dey SA, Karan S, De SK (2009) Solid State Commun 149:1282–1287
Chazeau L, Cavaillé JY, Perez J (2000) J Polym Sci: Part B: Polym Phys 38:383–392
Gopalan NK, Dufresne A (2003) Biomacromolecules 4: 657 – 656
Mohamad AA, Mohamad NS, Yahya MZA, Othman R, Ramesh S, Alias Y, Arof AK (2003) Solid State Ion 156:171–177
Yoshihara T, Tadokoro H, Murahashi S (1964) J Chem Phys 41:2902–2911
Ahmad S, Bohidar HB, Ahmad S, Agnihotry SA (2006) Polymer 47:3583–3590
Sireerat I (2005) ‘Preparation, characterization and molecular modelling of poly(ethyleneoxide)/poly(vinylpyrrolidone) montmorillonitenano- composite solid electrolytes’ Thesis adviser: Asst. Prof. Visit Vao-Soongnern, pp.54–175, ISBN:974–533–520–7
Hancock BC, Zografi G (1997) J Pharm Sci 86:1–12
Fragiadakis D, Pissis P (2007) J Non-Cryst Solids 353:4344–4352
Bandara TMWJ, Mellander B-E, Albinsson I, Dissanayake MAKL, Pitawala HMJC (2009) J Solid State Electrochem 13:1227–1232
Groenewoud WM (2001) Characterization of polymers by thermal analysis. Elsevier, Netherlands
Kim KM, Ryu KS, Kang SG, Chang SH, Chung IJ (2001) Macromol Chem Phy 202:866–872
Cordoba – Torres P, Mesquita TJ, Nogueira R (2015) J Phys Chem C 119:4136–4147
Kumar KK, Ravi M, Pavani Y, Bhavani S, Sharma AK, Rao VVRN (2014) J Membr Sci 454:200–211
Monisha S, Selvasekarapandian S, Mathavan T, Benial AMF, Manoharan S, Karthikeyan S (2016) J Mater Sci: Mater Electron 27:9314–9324
Ramalingaiah S, Sinivas Reddy S, Jaipal Reddy M, Laxminarsaiah E, Subba Rao UV (1996) Mater Lett 29:285–289
Jaipal Reddy M, Peter PC (2002) J Power Sources 109:340–346
Vincent C (1995) Electrochim Acta 40:2035–2040
Yang LL, McGhie F (1986) J Electrochem Soc 133:1380–1385
Karmakar A, Ghosha A (2014) AIP Adv 4: 087112
Chen-Yang YW, Chen YT, H. Chen HC, Lin WT, Tsai CH (2009) Polymer 50: 2856 – 2862
Wieczorek W (1992) Mater Sci Eng: B 15:108–114
Pas SJ, Ingram MD, Funke K, Hill AJ (2005) Electrochim Acta 50:3955–3962
Fergus JW (2010) J Power Sources 195:4554–4569
Sorensen TS, Compan V (1995) J Chem Soc Faraday Trans 91:4235–4250
Kumar M, Srivastava N (2014) J Non-Cryst Solids 389:28–34
Wang Y (2016) Ionic transport and dielectric relaxation in polymer electrolytes. In: Paluch M. (eds) Dielectric Properties of Ionic Liquids. Advances in Dielectrics. Springer, Cham
Kumar M, Srivastava N (2015) Ionics 21:1301–1310
Michael W, van Ernout M, K, John C, Jansen, Wim M, Jan van T, (1997) Macro. Rapid Commun 18:139–147
Wang W, Alexandridis P (2016) Polymers 8:387
Munar A, Andrio A, Iserte R, Compañ V (2011) J Non-Cryst Solids 357:3064–3069
Hidaya N, Nasir A, Chan CH, Kammer H-W, Sim LH, Yahya MZA (2010) Macromol Symp 290:46–55
White RP, Lipson JEG (2016) Macromolecules 49:3987–4007
Acknowledgements
Authors acknowledge the financial support by the BNSF of Bulgaria, under National Scientific Program “Petar Beron i NIE” (P. Beron) contract № KP-06-DB-1/16.12.2019. Authors express their sincere thanks to Dr. Vanna Sanna (Nanomater S.r.l.—Alghero (Sassary)—ITALY) for her kind support by providing starch nanocrystals of size about 80 nm and for providing SEM images to interpret the surface morphology of acid hydrolysed starch nanocrystals.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Koduru, H.K., Marinov, Y.G., Kaleemulla, S. et al. Fabrication and characterization of magnesium—ion-conducting flexible polymer electrolyte membranes based on a nanocomposite of poly(ethylene oxide) and potato starch nanocrystals. J Solid State Electrochem 25, 2409–2428 (2021). https://doi.org/10.1007/s10008-021-05018-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-05018-5