Abstract
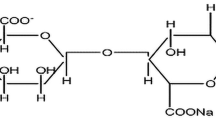
Herein, solid biopolymer electrolyte membranes based on sodium alginate are prepared and investigated for their application in sodium-ion batteries. Various concentrations of sodium thiocyanate (NaSCN) are introduced into the matrix of sodium alginate biopolymer. Solution cast route is the method opted to prepare the electrolyte membrane. The complex formation between sodium alginate and NaSCN has been confirmed with the help of X-ray diffraction (XRD) analysis and Fourier transform infrared spectroscopy (FTIR). On increasing NaSCN concentration, the semi-crystalline nature of the sodium alginate gets abated thus elevating the amorphous domain of the electrolyte membrane. Information about the glass transition temperature (Tg) is acquired from differential scanning calorimetry (DSC). Decrement in the Tg upon NaSCN addition favors the segmental motion of the polymer chain. The biopolymer host material (30 wt%) can accommodate large amounts of NaSCN salt (70 wt%) exhibiting ionic conductivity of 1.22 × 10−2 S cm−1. The transference number measurement with Wagner’s DC polarization method is found to be 0.96 (near unity) which confirms ions are the governing charge carriers. The linear sweep voltammetry (LSV) technique that measures the potential window for the biopolymer electrolyte membrane is 2.7 V, representing it as a potential applicant for electrochemical energy storage devices. An all-solid-state sodium-ion battery is assembled with a high ion–conducting biopolymer electrolyte membrane that displays an open cell potential of 2.87 V. The results highlight the possibilities of sodium ion–conducting solid biopolymer electrolytes to extend their hands in a safe sodium-ion battery.









Similar content being viewed by others
References
Vikström H, Davidsson S, Höök M (2013) Lithium availability and future production outlooks. Appl Energy 110:252–266
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114:11636–11682
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5:5884–5901
Che H, Chen S, Xie Y, Wang H, Amine K, Liao XZ, Ma ZF (2017) Electrolyte design strategies and research progress for room-temperature sodium-ion batteries. Energy Environ Sci 10:1075–1101
Kim JJ, Yoon K, Park I, Kang K (2017) Progress in the development of sodium-ion solid electrolytes. Small Methods 1:1700219
Farrington GC, Dunn B (1982) Divalent beta″-aluminas: high conductivity solid electrolytes for divalent cations. Solid State Ionics 7:267–281
Song S, Duong HM, Korsunsky AM, Hu N, Lu L (2016) A Na+ superionic conductor for room-temperature sodium batteries. Sci Rep 6:1–10
Koduru HK, Iliev MT, Kondamareddy KK, Karashanova D, Vlakhov T, Zhao XZ, Scaramuzza N (2016) Investigations on poly (ethylene oxide) (PEO) - blend based solid polymer electrolytes for sodium ion batteries. J Phys Conf Ser 764:1–10
Manoravi P, Selvaraj II, Chandrasekhar V, Shahi K (1993) Conductivity studies of new polymer electrolytes based on the poly(ethylene glycol)/sodium iodide system. Polymer 34:1339–1341
Naresh Kumar K, Sreekanth T, Jaipal Reddy M, Subba Rao UV (2001) Study of transport and electrochemical cell characteristics of PVP:NaClO3 polymer electrolyte system. J Power Sources 101:130–133
Mindemark J, Mogensen R, Smith MJ, Silva MM, Brandell D (2017) Polycarbonates as alternative electrolyte host materials for solid-state sodium batteries. Electrochem Commun 77:58–61
Zhou D, Liu R, Zhang J, Qi X, He YB, Li B et al (2017) In situ synthesis of hierarchical poly(ionic liquid)-based solid electrolytes for high-safety lithium-ion and sodium-ion batteries. Nano Energy 33:45–54
Subba Reddy CV, Jin AP, Zhu QY, Mai LQ, Chen W (2006) Preparation and characterization of (PVP + NaClO4) electrolytes for battery applications. Eur Phys J E 19:471–476
Aziz SB, Hamsan MH, Kadir MFZ, Karim WO, Abdullah RM (2019) Development of polymer blend electrolyte membranes based on chitosan: dextran with high ion transport properties for EDLC Application. Int J Mol Sci 20(13):3369
Kim JH, Min BR, Won J, Kim CK, Kang YS (2004) Structure and coordination properties of facilitated olefin transport membranes consisting of crosslinked poly(vinyl alcohol) and silver hexafluoroantimonate. J Polym Sci Part B Polym Phys 42:621–628
Gong SD, Huang Y, Cao HJ, Lin YH, Li Y, Tang SH, Wang MS, Li X (2016) A green and environment-friendly gel polymer electrolyte with higher performances based on the natural matrix of lignin. J Power Sources 307:624–633
Zhang J, Yue L, Kong Q, Liu Z, Zhou X, Zhang C, Xu Q, Zhang B, Ding G, Qin B, Duan Y, Wang Q, Yao J, Cui G, Chen L (2014) Sustainable, heat-resistant and flame-retardant cellulose-based composite separator for high-performance lithium ion battery. Sci Rep 4:1–8
Ng LS, Mohamad AA (2008) Effect of temperature on the performance of proton batteries based on chitosan-NH4NO3-EC membrane. J Memb Sci 325:653–657
Samsudin AS, Lai HM, Isa MIN (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Shukur MF, Kadir MFZ (2014) Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 21:111–124
Huang Y, Liu J, Zhang J, Jin S, Jiang Y, Zhang S, Li Z, Zhi C, Du G, Zhou H (2019) Flexible quasi-solid-state zinc ion batteries enabled by highly conductive carrageenan bio-polymer electrolyte. RSC Adv 9:16313–16319
Singh R, Bhattacharya B, Tomar SK, Singh V, Singh PK (2017) Electrical, optical and electrophotochemical studies on agarose based biopolymer electrolyte towards dye sensitized solar cell application. Measurement 102:214–219
Mindemark J, Lacey MJ, Bowden T, Brandell D (2018) Beyond PEO—alternative host materials for Li+-conducting solid polymer electrolytes. Prog Polym Sci 81:114–143
Zhang L, Liu Z, Cui G, Chen L (2015) Biomass-derived materials for electrochemical energy storages. Prog Polym Sci 43:136–164
Kovalenko I, Zdyrko B, Magasinski A, Hertzberg B, Milicev Z, Burtovyy R, Luzinov I, Yushin G (2011) A major constituent of brown algae for use in high-capacity Li-ion batteries. Science 334(6052):75–79
Ling L, Bai Y, Wang Z, Ni Q, Chen G, Zhou Z, Wu C (2018) Remarkable effect of sodium alginate aqueous binder on anatase TiO2 as high-performance anode in sodium ion batteries. ACS Appl Mater Interfaces 10:5560–5568
Smitha B, Sridhar S, Khan AA (2005) Chitosan-sodium alginate polyion complexes as fuel cell membranes. Eur Polym J 41:1859–1866
Yang JM, Wang NC, Chiu HC (2014) Preparation and characterization of poly(vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J Memb Sci 457:139–148
Kadokawa JI, Saitou S, Shoda SI (2005) Preparation of alginate-polymethacrylate hybrid material by radical polymerization of cationic methacrylate monomer in the presence of sodium alginate. Carbohydr Polym 60:253–258
Hu O, Chen G, Gu J, Lu J, Zhang J, Zhang X, Hou L, Jiang X (2020) A facile preparation method for anti-freezing, tough, transparent, conductive and thermoplastic poly(vinyl alcohol)/sodium alginate/glycerol organohydrogel electrolyte. Int J Biol Macromol 164:2512–2523
Czubacka E, Kruszynski R, Sieranski T (2012) The structure and thermal behaviour of sodium and potassium multinuclear compounds with hexamethylenetetramine. Struct Chem 23:451–459
Zhu H, Ströbele M, Yu Z, Wang Z, Meyer HJ, You X (2001) A blue luminescent di-2-pyridylamine cadmium complex with an unexpected arrangement of thiocyanate ligands: a supramolecular layered structure based on hydrogen bonds and π-π stacking interactions. Inorg Chem Commun 4:577–581
Chikte (Awade) D, Omanwar SK (2019) Synthesis and luminescence properties of novel NaSCN: xCe3+ phosphor. J Asian Ceramic Soc 7:350–354
Wan Y, Creber KAM, Peppley B, Tam Bui V (2006) Chitosan-based solid electrolyte composite membranes. I Preparation and characterization. J Memb Sci 280:666–674
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37:1371–1376
Malathi J, Kumaravadivel M, Brahmanandhan GM, Hema M, Baskaran R, Selvasekarapandian S (2010) Structural, thermal and electrical properties of PVA-LiCF3SO3 polymer electrolyte. J Non Cryst Solids 356:2277–2281
Aziz SB (2016) Role of dielectric constant on ion transport: reformulated Arrhenius equation. Adv Mater Sci Eng 2527013. https://doi.org/10.1155/2016/2527013
Hashmi SA, Chandra S (1995) Experimental investigations on a sodium-ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. Mater Sci Eng B 34:18–26
Sanders RA, Snow AG, Frech R, Glatzhofer DT (2003) A spectroscopic and conductivity comparison study of linear poly(N-methylethylenimine) with lithium triflate and sodium triflate. Electrochim Acta 48:2247–2253
Fuzlin AF, Samsudin AS (2020) Studies on favorable ionic conduction and structural properties of biopolymer electrolytes system-based alginate. Polym Bull. https://doi.org/10.1007/s00289-020-03207-2
Choi S, Kim JH, Kang YS (2001) Wide-angle X-ray scattering studies on the structural properties of polymer electrolytes containing silver ions. Macromolecules 34:9087–9092
Bhajantri RF, Ravindrachary V, Harisha A, Ranganathaiah C, Kumaraswamy GN (2007) Effect of barium chloride doping on PVA microstructure: positron annihilation study. Appl Phys A Mater Sci Process 87:797–805
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Pandi DV, Nithya S, Sanjeeviraja C (2015) Electrical conductivity characterization of polyacrylonitrile-ammonium bromide polymer electrolyte system. J Solid State Electrochem 19:987–999
Fuzlin AF, Bakri NA, Sahraoui B, Samsudin AS (2020) Study on the effect of lithium nitrate in ionic conduction properties based alginate biopolymer electrolytes. Mater Res Express 7(1):015902
Fromm KM (2008) Coordination polymer networks with s-block metal ions. Coord Chem Rev 252:856–885
Rivas BL, Maureira AE, Mondaca MA (2008) Aminodiacetic water-soluble polymer-metal ion interactions. Eur Polym J 44:2330–2338
De Santana H, Pelisson L, Diogo RJ, Zaia CTBV, Zaia DAM (2010) UV Radiation and the reaction between ammonium and thiocyanate under prebiotic chemistry conditions. J Serb Chem Soc 75:1381–1389
Salleh NS, Aziz SB, Aspanut Z, Kadir MFZ (2016) Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 22:2157–2167
Ismayil VR, Bhajantri RF, Dhola PS, Sanjeev G (2015) Impact of electron-beam irradiation on free-volume related microstructural properties of PVA:NaBr polymer composites. Nucl Instruments Methods Phys Res Sect B Beam Interact with Mater Atoms 342:29–38
Mazuki NF, Fuzlin AF, Saadiah MA, Samsudin AS (2019) An investigation on the abnormal trend of the conductivity properties of CMC/PVA-doped NH4Cl-based solid biopolymer electrolyte system. Ionics 25:2657–2667
Kadir MFZ, Salleh NS, Hamsan MH, Aspanut Z, Majid NA, Shukur MF (2018) Biopolymeric electrolyte based on glycerolized methyl cellulose with NH4Br as proton source and potential application in EDLC. Ionics 24:1651–1662
Nakamura K, Nishimura Y, Hatakeyama T, Hatakeyama H (1995) Thermal properties of water insoluble alginate films containing di- and trivalent cations. Thermochim Acta 267:343–353
Siddaramaiah STMM, Ramaraj B, Lee JH (2008) Sodium alginate and its blends with starch: Thermal and morphological properties. J Appl Polym Sci 109:4075–4081
Swamy TMM, Ramaraj B, Siddaramaiah (2010) Sodium alginate and poly(ethylene glycol) blends: thermal and morphological behaviors. J Macromol Sci Part A Pure Appl Chem 47:877–881
Ojovan MI (2008) Viscosity and glass transition in amorphous oxides. Adv Condens Matter Phys 2008:1–23
Kim JH, Min BR, Won J, Kang YS (2003) Analysis of the glass transition behavior of polymer−salt complexes an extended configurational entropy model. J Phys Chem B 107(24):5901–5905
Chandra MVL, Karthikeyan S, Selvasekarapandian S, Pandi DV, Monisha S, Packiaseeli SA (2016) Characterization of high ionic conducting PVAc–PMMA blend-based polymer electrolyte for electrochemical applications. Ionics 22:2409–2420
Mohapatra SR, Thakur AK, Choudhary RNP (2009) Effect of nanoscopic confinement on improvement in ion conduction and stability properties of an intercalated polymer nanocomposite electrolyte for energy storage applications. J Power Sources 191:601–613
Sundaramahalingam K, Muthuvinayagam M, Nallamuthu N (2019) AC impedance analysis of lithium ion based PEO:PVP solid polymer blend electrolytes. Polym Sci Ser A 61:565–576
Boukamp BA (1986) A nonlinear least squares fit procedure for analysis of immittance data of electrochemical systems. Solid State Ionics 20:31–44
Saiful M, Rani A, Mohammad M, Sukor M, Ahmad A, Mohamed NS (2020) Novel approach for the utilization of ionic liquid - based cellulose derivative biosourced polymer electrolytes in safe sodium - ion batteries. Polym Bull. https://doi.org/10.1007/s00289-020-03382-2
Wagner JB, Wagner C (1957) Electrical conductivity measurements on cuprous halides. J Chem Phys 26:1597–1601
Okamoto Y, Tsuzuki S, Tatara R, Ueno K, Dokko K, Watanabe M (2020) High transference number of Na ion in liquid-state sulfolane solvates of sodium Bis(fluorosulfonyl)amide. J Phys Chem C 124(8):4459–4469
Slane S, Salomon M (1995) Composite gel electrolyte for rechargeable lithium batteries. J Power Sources 55:7–10
Ibrahim S, Ahmad A, Mohamed NS (2015) Characterization of novel castor oil-based polyurethane polymer electrolytes. Polymers 7:747–759
Isa KBM, Othman L, Hambali D, Osman Z (2017) Electrical and electrochemical studies on sodium ion-based gel polymer electrolytes. AIP Conf Proc 1877:040001
Subba Reddy CV, Sharma AK, Narasimha Rao VVR (2003) Conductivity and discharge characteristics of polyblend (PVP + PVA + KIO3) electrolyte. J Power Sources 114:338–345
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diana, M.I., Selvin, P.C., Selvasekarapandian, S. et al. Investigations on Na-ion conducting electrolyte based on sodium alginate biopolymer for all-solid-state sodium-ion batteries. J Solid State Electrochem 25, 2009–2020 (2021). https://doi.org/10.1007/s10008-021-04985-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-021-04985-z