Abstract
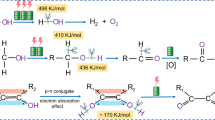
Photoelectrochemical (PEC) oxidation of biomass is a profitable approach to produce hydrogen by substituting the water oxidation reaction in the electrolyzers’ photoanodes. Among the biomass-derived molecules, glycerol is an interesting alternative to water since its standard thermodynamic potential is considerably lower than that of water and because it is widely produced in the biodiesel industry. Herein, we performed a fundamental study of the PEC oxidation of glycerol on hematite. In situ FTIR experiments and long-term electrolysis followed by HPLC analysis revealed C1, C2 and C3 oxidation products showing the low selectivity of the reaction under these conditions. We explained this lack of selectivity by an electrooxidation mechanism involving highly reactive radicals as intermediates.

Graphical abstract






Similar content being viewed by others
References
Ibrahim N, Kamarudin SK, Minggu LJ (2014) Biofuel from biomass via photo-electrochemical reactions: an overview. J Power Sources 259:33–42
Pawar RC, Pyo Y, Ahn SH, Lee CS (2015) Photoelectrochemical properties and photodegradation of organic pollutants using hematite hybrids modified by gold nanoparticles and graphitic carbon nitride. Appl Catal B Environ 176–177:654–666
Li G, Wang C, Yan Y, Yan X, Li W, Feng X, Li J, Xiang Q, Tan W, Liu F, Yin H (2020) Highly enhanced degradation of organic pollutants in hematite/sulfite/photo system. Chem Eng J 386:124007
Mesa CA, Kafizas A, Francàs L, Pendlebury SR, Pastor E, Ma Y, le Formal F, Mayer MT, Grätzel M, Durrant JR (2017) Kinetics of photoelectrochemical oxidation of methanol on hematite photoanodes. J Am Chem Soc 139(33):11537–11543
Kalamaras E, Dracopoulos V, Sygellou L, Lianos P (2016) Electrodeposited Ti-doped hematite photoanodes and their employment for photoelectrocatalytic hydrogen production in the presence of ethanol. Chem Eng J 295:288–294
Wang G, Ling Y, Lu X, Zhai T, Qian F, Tong Y, Li Y (2013) A mechanistic study into the catalytic effect of Ni(OH)2 on hematite for photoelectrochemical water oxidation. Nanoscale 5(10):4129–4133
Shimura K, Yoshida H (2011) Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ Sci 4(7):2467–2481
Reichert R, Zambrzycki C, Jusys Z, Behm RJ (2015) Photo-electrochemical oxidation of organic C1 molecules over WO3 films in aqueous electrolyte: competition between water oxidation and C1 oxidation. ChemSusChem 8(21):3677–3687
Monllor-Satoca D, Borja L, Rodes A, Gómez R, Salvador P (2006) Photoelectrochemical behavior of nanostructured WO3 thin-film electrodes: the oxidation of formic acid. ChemPhysChem 7(12):2540–2551
Lu X, Xie S, Yang H, Tong Y, Ji H (2014) Photoelectrochemical hydrogen production from biomass derivatives and water. Chem Soc Rev 43(22):7581–7593
Ru Ng AY, Boruah B, Chin KF, Modak JM, Soo HS (2020) Photoelectrochemical cells for artificial photosynthesis: alternatives to water oxidation. ChemNanoMat 6(2):185–203
Pagliaro M, Ciriminna R, Kimura H, Rossi M, Della Pina C (2007) From glycerol to value-added products. Angew Chemie Int Ed 46:4434–4440
Bagheri S, Julkapli NM, Yehye WA (2015) Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew Sust Energ Rev 41:113–127
Martins CA, Fernández PS, Camara GA (2018) Alternative uses for biodiesel byproduct: glycerol as source of energy and high valuable chemicals. Springer, Cham, pp 159–186
Coutanceau C, Baranton S, Kouamé RSB (2019) Selective electrooxidation of glycerol into value-added chemicals: a short overview. Front Chem 7:1–15
Ibadurrohman M, Hellgardt K (2020) Importance of surface roughness of TiO2 photoanodes in promoting photoelectrochemical activities with and without sacrificial agent. Thin Solid Films 705:138009
Bashiri R, Mohamed NM, Sufian S, Kait CF (2020) Improved photoelectrochemical hydrogen production over decorated titania with copper and nickel oxides by optimizing the photoanode and reaction characteristics. Mater Today Chem 16:100241
Seadira TWP, Masuku CM, Scurrell MS (2020) Solar photocatalytic glycerol reforming for hydrogen production over ternary cu/THS/graphene photocatalyst: effect of Cu and graphene loading. Renew Energy 156:84–97
Ravi P, Navakoteswara Rao V, Shankar MV, Sathish M (2020) CuO@NiO core-shell nanoparticles decorated anatase TiO2 nanospheres for enhanced photocatalytic hydrogen production. Int J Hydrog Energy 45(13):7517–7529
Liu D, Liu J-C, Cai W, Ma J, Yang HB, Xiao H, Li J, Xiong Y, Huang Y, Liu B (2019) Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat Commun 10(1):1779
Huang L-W, Vo T-G, Chiang C-Y (2019) Converting glycerol aqueous solution to hydrogen energy and dihydroxyacetone by the BiVO4 photoelectrochemical cell. Electrochim Acta 322:134725
Souza FL (2018) Sunlight-driven water splitting using hematite nanorod photoelectrodes. An Acad Bras Cienc 90:745–762
Jiang C, Moniz SJA, Wang A, Zhang T, Tang J (2017) Photoelectrochemical devices for solar water splitting – materials and challenges. Chem Soc Rev 46(15):4645–4660
Muche DNF, dos Santos TMG, Leite GP, Melo MA Jr, Gonçalves RV, Souza FL (2019) Tailoring hematite/FTO interfaces: new horizons for spin-coated hematite photoanodes targeting water splitting. Mater Lett 254:218–221
Dharmadasa IM, Haigh J (2006) Strengths and advantages of electrodeposition as a semiconductor growth technique for applications in macroelectronic devices. J Electrochem Soc 153(1):G47
Jang JT, Ryu H, Lee WJ (2015) The growth of hematite by electrochemical deposition for PEC applications. J Alloys Compd 638:387–392
Liang P, Li L, Liu C, Wang W, Zhang H, Mitsuzaki N, Chen Z (2018) Effects of cathodic electrodeposition conditions on morphology and photoelectrochemical response of α-Fe2O3 photoanode. Thin Solid Films 666:161–171
Chong R, Wang B, Li D, Chang Z, Zhang L (2017) Enhanced photoelectrochemical activity of nickel-phosphate decorated phosphate-Fe2O3photoanode for glycerol-based fuel cell. Sol Energy Mater Sol Cells 160:287–293
Bott-Neto JL, Rodrigues MVF, Silva MC, Carneiro-Neto EB, Wosiak G, Mauricio JC, Pereira EC, Figueroa SJA, Fernández PS (2020) Versatile spectroelectrochemical cell for in situ experiments: development, applications, and electrochemical behavior. ChemElectroChem 7(21):4306–4313
Shinde PS, Annamalai A, Kim JH, Choi SH, Lee JS, Jang JS (2015) Exploiting the dynamic Sn diffusion from deformation of FTO to boost the photocurrent performance of hematite photoanodes. Sol Energy Mater Sol Cells 141:71–79
de Faria DLA, Lopes FN (2007) Heated goethite and natural hematite: can Raman spectroscopy be used to differentiate them? Vib Spectrosc 45(2):117–121
Cherepy NJ, Liston DB, Lovejoy JA, Deng H, Zhang JZ (1998) Ultrafast studies of photoexcited electron dynamics in γ- and α-Fe2O3 semiconductor nanoparticles. J Phys Chem B 102(5):770–776
Townsend TK, Sabio EM, Browning ND, Osterloh FE (2011) Photocatalytic water oxidation with suspended alpha-Fe2O3 particles-effects of nanoscaling. Energy Environ Sci 4(10):4270–4275
Iandolo B, Zhang H, Wickman B, Zorić I, Conibeer G, Hellman A (2015) Correlating flat band and onset potentials for solar water splitting on model hematite photoanodes. RSC Adv 5(75):61021–61030
Carvalho-Jr WM, Souza FL (2016) Thermal enhancement of water affinity on the surface of undoped hematite photoelectrodes. Sol Energy Mater Sol Cells 144:395–404
Tilley SD, Cornuz M, Sivula K, Grätzel M (2010) Light-induced water splitting with hematite: improved nanostructure and iridium oxide catalysis. Angew Chemie Int Ed 49(36):6405–6408
Le Formal F, Pastor E, Tilley SD et al (2015) Rate law analysis of water oxidation on a hematite surface. J Am Chem Soc 137(20):6629–6637
Rohloff M, Cosgun S, Massué C, Lunkenbein T, Senyshyn A, Lerch M, Fischer A, Behrens M (2019) The role of synthesis conditions for structural defects and lattice strain in β-TaON and their effect on photo- and photoelectrocatalysis. Zeitschrift für Naturforsch B 74(1):71–83
Cesar I, Sivula K, Kay A, Zboril R, Grätzel M (2009) Influence of feature size, film thickness, and silicon doping on the performance of nanostructured hematite photoanodes for solar water splitting. J Phys Chem C 113(2):772–782
Ling Y, Wang G, Wheeler DA, Zhang JZ, Li Y (2011) Sn-doped hematite nanostructures for photoelectrochemical water splitting. Nano Lett 11(5):2119–2125
Korjenic A, Raja KS (2019) Electrochemical stability of fluorine doped tin oxide (FTO) coating at different pH conditions. J Electrochem Soc 166(6):C169–C184
de Souza MBC, Yukuhiro VY, Vicente RA, Vilela Menegaz Teixeira Pires CTG, Bott-Neto JL, Fernández PS (2020) Pb- and bi-modified Pt electrodes toward glycerol electrooxidation in alkaline media. Activity, selectivity, and the importance of the Pt atoms arrangement. ACS Catal 10(3):2131–2137
Jeffery DZ, Camara GA (2010) The formation of carbon dioxide during glycerol electrooxidation in alkaline media: first spectroscopic evidences. Electrochem Commun 12(8):1129–1132
De Souza MBC, Vicente RA, Yukuhiro VY et al (2019) Bi-modified Pt electrodes toward glycerol electrooxidation in alkaline solution: effects on activity and selectivity. ACS Catal 9(6):5104–5110
Lima CC, Rodrigues MVF, Neto AFM, Zanata CR, Pires CTGVMT, Costa LS, Solla-Gullón J, Fernández PS (2020) Highly active Ag/C nanoparticles containing ultra-low quantities of sub-surface Pt for the electrooxidation of glycerol in alkaline media. Appl Catal B Environ 279:119369
Lux S, Siebenhofer M (2013) Synthesis of lactic acid from dihydroxyacetone: use of alkaline-earth metal hydroxides. Catal Sci Technol 3(5):1380–1385
Birdja YY, Koper MTM (2017) The importance of cannizzaro-type reactions during electrocatalytic reduction of carbon dioxide. J Am Chem Soc 139(5):2030–2034
Tamirat AG, Rick J, Dubale AA, Su WN, Hwang BJ (2016) Using hematite for photoelectrochemical water splitting: a review of current progress and challenges. Nanoscale Horizons 1(4):243–267
Reichert R, Jusys Z, Behm RJ (2014) A novel photoelectrochemical flow cell with online mass spectrometric detection: oxidation of formic acid on a nanocrystalline TiO2 electrode. Phys Chem Chem Phys 16(45):25076–25080
Di Valentin C, Fittipaldi D (2013) Hole scavenging by organic adsorbates on the TiO2 surface: a DFT model study. J Phys Chem Lett 4(11):1901–1906
Habibi E, Razmi H (2012) Glycerol electrooxidation on Pd, Pt and Au nanoparticles supported on carbon ceramic electrode in alkaline media. Int J Hydrog Energy 37(22):16800–16809
Mao Z, Campbell CT (2019) Apparent activation energies in complex reaction mechanisms: a simple relationship via degrees of rate control. ACS Catal 9(10):9465–9473
Acknowledgements
The authors gratefully acknowledge the support from the FAPESP (Sao Paulo Research Foundation): (grants: #2013/07296-2, 2016/01365-0, 2017/11986-5, 2018/20952-0, 2019/07449-0), Brazilian Council for Scientific and Technological Development (CNPq) (Grant #430426/2018-6) and Shell and the strategic importance of the support given by the ANP (Brazilian National Oil, Natural Gas and Biofuels Agency) through the R&D levy regulation. This study was also financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1145 kb)
Rights and permissions
About this article
Cite this article
Perini, N., Hessel, C., Bott-Neto, J.L. et al. Photoelectrochemical oxidation of glycerol on hematite: thermal effects, in situ FTIR and long-term HPLC product analysis. J Solid State Electrochem 25, 1101–1110 (2021). https://doi.org/10.1007/s10008-020-04878-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04878-7