Abstract
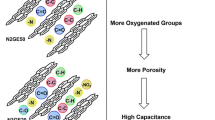
The restacking of graphene layers in chemically synthesized few-layered graphene can be reduced through approaches such as nitrogen doping, introduction of spacer materials in-between the graphene layers, and compositing with metal oxides. In the present study, graphene oxide (GO) is synthesized by a modified Hummers’ method and reduced hydrothermally using different amounts of commercial protein powder, protineX. Also, GO is reduced hydrothermally in the absence of protineX for the purpose of comparison. ProtineX not only serves as a nitrogen dopant, but also as a precursor for the carbonaceous spacer. The effect of the presence of protineX during hydrothermal reduction of GO on the purity, amount of nitrogen doped, and the capacitance properties of RGO is systematically studied via various physico-chemical and electrochemical methods. On increasing the concentration of protineX during hydrothermal reduction of GO, the percentage of nitrogen doped in RGO and the surface area are found to increase. The specific capacitance of RGO is found to increase gradually from 115 F g−1 to 235 F g−1 at a current density of 1 A g−1 on increasing the concentration of protineX from 0 to 20 wt% during the hydrothermal reduction of GO. On increasing the concentration of protineX further, the specific capacitance value of RGO is found to decrease.






Similar content being viewed by others
References
Najib S, Erdem E (2019) Current progress achieved in novel materials for supercapacitor electrodes: mini review. Nanoscale Adv 1:2817–2827
Conway BE (1991) Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage. J Electrochem Soc 138:1539–1548
Frackowiak E (2007) Carbon materials for supercapacitor application. Phys Chem Chem Phys 9(15):1774–1785
Genc R, Alas MO, Harputlu E et al (2017) High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci Rep 7:1–13
Kasap S, Kaya II, Repp S, Erdem E (2019) Superbat: battery-like supercapacitor utilized by graphene foam and zinc oxide (ZnO) electrodes induced by structural defects. Nanoscale Adv 1:2586–2597
Yang Z, Tian J, Yin Z et al (2019) Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: a review. Carbon 141:467–480
Wang J, Li Q, Peng C et al (2020) To increase electrochemical performance of electrode material by attaching activated carbon particles on reduced graphene oxide sheets for supercapacitor. J Power Sources 450:227611
Boukhalfa S, Evanoff K, Yushin G (2012) Atomic layer deposition of vanadium oxide on carbon nanotubes for high-power supercapacitor electrodes. Energy Environ Sci 5:6872–6879
Tang Z, Tang C, Gong H (2012) A high energy density asymmetric supercapacitor from nano-architectured Ni (OH) 2/carbon nanotube electrodes. Adv Funct Mater 22:1272–1278
Liu C, Yu Z, Neff D, Zhamu A, Jang BZ (2010) Graphene-based supercapacitor with an ultrahigh energy density. Nano Lett 10(12):4863–4868
Mayorov AS, Gorbachev RV, Morozov SV, Britnell L, Jalil R, Ponomarenko LA, Blake P, Novoselov KS, Watanabe K, Taniguchi T, Geim AK (2011) Micrometer-scale ballistic transport in encapsulated graphene at room temperature. Nano Lett 11(6):2396–2399
Hummers WS Jr, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339–1339
Wang Q, Yan J, Fan Z et al (2014) Mesoporous polyaniline film on ultra-thin graphene sheets for high performance supercapacitors. J Power Sources 247:197–203
Kumar NA, Choi H-J, Shin YR, Chang DW, Dai L, Baek JB (2012) Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano 6(2):1715–1723
Lu X, Dou H, Gao B et al (2011) A flexible graphene/multiwalled carbon nanotube film as a high performance electrode material for supercapacitors. Electrochim Acta 56:5115–5121
Lee SU, Belosludov RV, Mizuseki H, Kawazoe Y (2009) Designing nanogadgetry for nanoelectronic devices with nitrogen-doped capped carbon nanotubes. Small 5(15):1769–1775
Fan W, Xia Y-Y, Tjiu WW et al (2013) Nitrogen-doped graphene hollow nanospheres as novel electrode materials for supercapacitor applications. J Power Sources 243:973–981
Yu D, Dai L (2010) Self-assembled graphene/carbon nanotube hybrid films for supercapacitors. J Phys Chem Lett 1:467–470
Wu Z-S, Zhou G, Yin L-C et al (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131
Lu T, Zhang Y, Li H et al (2010) Electrochemical behaviors of graphene–ZnO and graphene–SnO2 composite films for supercapacitors. Electrochim Acta 55:4170–4173
Yan J, Wei T, Shao B et al (2010) Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon 48:1731–1737
Cheng Q, Tang J, Ma J et al (2011) Graphene and carbon nanotube composite electrodes for supercapacitors with ultra-high energy density. Phys Chem Chem Phys 13:17615–17624
Choi H-J, Jung S-M, Seo J-M et al (2012) Graphene for energy conversion and storage in fuel cells and supercapacitors. Nano Energy 1:534–551
Zhang C, Lv W, Xie X et al (2013) Towards low temperature thermal exfoliation of graphite oxide for graphene production. Carbon 62:11–24
Yang S, Zhi L, Tang K et al (2012) Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reactions. Adv Funct Mater 22:3634–3640
Choi CH, Chung MW, Park SH, Woo SI (2013) Additional doping of phosphorus and/or sulfur into nitrogen-doped carbon for efficient oxygen reduction reaction in acidic media. Phys Chem Chem Phys 15(6):1802–1805
Ferrari AC, Meyer JC, Scardaci V et al (2006) Raman spectrum of graphene and graphene layers. Phys Rev Lett 97:187401
Cançado LG, Reina A, Kong J, Dresselhaus MS (2008) Geometrical approach for the study of G′ band in the Raman spectrum of monolayer graphene, bilayer graphene, and bulk graphite. Phys Rev B 77:245408
Kaniyoor A, Ramaprabhu S (2012) A Raman spectroscopic investigation of graphite oxide derived graphene. AIP Adv 2:032183
Pham CV, Krueger M, Eck M et al (2014) Comparative electron paramagnetic resonance investigation of reduced graphene oxide and carbon nanotubes with different chemical functionalities for quantum dot attachment. Appl Phys Lett 104:132102
Pels JR, Kapteijn F, Moulijn JA et al (1995) Evolution of nitrogen functionalities in carbonaceous materials during pyrolysis. Carbon 33:1641–1653
Matter PH, Zhang L, Ozkan US (2006) The role of nanostructure in nitrogen-containing carbon catalysts for the oxygen reduction reaction. J Catal 239:83–96
Deng D, Pan X, Yu L et al (2011) Toward N-doped graphene via solvothermal synthesis. Chem Mater 23:1188–1193
Liu R, Duay J, Lane T, Lee SB (2010) Synthesis and characterization of RuO 2/poly (3, 4-ethylenedioxythiophene) composite nanotubes for supercapacitors. Phys Chem Chem Phys 12(17):4309–4316
Xu J, Wang K, Zu S-Z, Han BH, Wei Z (2010) Hierarchical nanocomposites of polyaniline nanowire arrays on graphene oxide sheets with synergistic effect for energy storage. ACS Nano 4(9):5019–5026
Gajjela SR, Ananthanarayanan K, Yap C et al (2010) Synthesis of mesoporous titanium dioxide by soft template based approach: characterization and application in dye-sensitized solar cells. Energy Environ Sci 3:838–845
Acknowledgments
We thank SASTRA for infrastructural and instrumental facilities. One of the authors (PB) acknowledges CSIR, India for senior research fellowship.
Funding
S.R.S. gratefully acknowledges financial support from the Science and Engineering Research Board, Department of Science and Technology, India (SB/FT/CS-070/2012).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 7672 kb)
Rights and permissions
About this article
Cite this article
Bharathidasan, P., Devaraj, S. & Sivakkumar, S.R. The capacitance properties of nitrogen doped reduced graphene oxide obtained by using commercial protein powder as a nitrogen dopant. J Solid State Electrochem 24, 1095–1103 (2020). https://doi.org/10.1007/s10008-020-04565-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04565-7