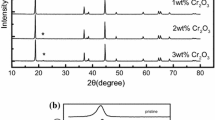
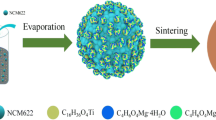
Abstract
In this paper, the dual-modified LiNi0.8Co0.1Mn0.1O2 via Gd2O3 is successfully obtained by the solid-state method. The phenomenon of Li/Ni cation mixing and structural stability of materials has been improved after Gd2O3 modification. Additionally, the lower average discharge voltage drop (ΔEd) and electrochemical polarization of the batteries are obtained after modification. Simultaneously, the dual-modified material via Gd2O3 exhibits the high capacity retention of 94.50% compared with that (83.40%) of the Pristine (3.0–4.4 V, 1 C (180 mA g−1), 25 °C, after 100 cycles). The excellent electrochemical properties ascribe to the higher bond dissociation energies of Gd-O (716 kJ mol−1) and the stable coating layer of Gd2O3.








Similar content being viewed by others
References
Meister P, Jia H, Li J et al (2016) Best practice: performance and cost evaluation of lithium ion battery active materials with special emphasis on energy efficiency. Chem Mater 28:7203–7217
Blomgren GE (2017) The development and future of lithium ion batteries. J Electrochem Soc 164:A5019–A5025
Placke T, Kloepsch R, Dühnen S, Winter M (2017) Lithium ion, lithium metal, and alternative rechargeable battery technologies: the odyssey for high energy density. J Solid State Electrochem 21:1939–1964
Zhu X, Yang J, Dastan D et al (2019) Fabrication of core-shell structured Ni@BaTiO3 scaffolds for polymer composites with ultrahigh dielectric constant and low loss. Compos Part A Appl Sci Manuf 125:105521
Lu H, Zhou H, Svensson AM et al (2013) High capacity Li [Ni0.8Co0.1Mn0.1]O2 synthesized by sol-gel and co-precipitation methods as cathode materials for lithium-ion batteries. Solid State Ionics 249-250:105–111
Xu J, Chen X, Wang C et al (2017) Nano-Y2O3-coated LiNi0.5Co0.2Mn0.3O2 cathodes with enhanced electrochemical stability under high cut-off voltage and high temperature. Ceram Int 43:11848–11854
Xu S, Du C, Xu X et al (2017) A mild surface washing method using protonated polyaniline for Ni-rich LiNi0.8Co0.1Mn0.1O2 material of lithium ion batteries. Electrochim Acta 248:534–540
Zhang B, Dong P, Tong H et al (2017) Enhanced electrochemical performance of LiNi0.8Co0.1Mn0.1O2with lithium-reactive Li3VO4coating. J Alloys Compd 706:198–204
Liu X, Liu J, Huang T, Yu A (2013) CaF2-coated Li1.2Mn0.54Ni0.13Co0.13O2as cathode materials for Li-ionbatteries. Electrochim Acta 109:52–58
Meng K, Wang Z, Guo H et al (2016) Improving the cycling performance of LiNi0.8Co0.1Mn0.1O2 by surface coating with Li2TiO3. Electrochim Acta 211:822–831
Chen D, Zheng F, Li L et al (2017) Effect of Li3PO4 coating of layered lithium-rich oxide on electrochemical performance. J Power Sources 341:147–155
Dai S, Yuan M, Wang L et al (2019) Ultrathin-Y2O3-coated LiNi0.8Co0.1Mn0.1O2 as cathode materials for Li-ion batteries: synthesis, performance and reversibility. Ceram Int 45:674–680
Li X, Zhang L, Ding Z, He Y (2017) Ultrafine Nd2O3nanoparticles doped carbon aerogel to immobilize sulfur for high performance lithium-sulfur batteries. J Electroanal Chem 799:617–624
Xia L, Qiu K, Gao Y, He X, Zhou F (2015) High potential performance of cerium-doped LiNi0.5Co0.2Mn0.3O2 cathode material for Li-ion battery. J Mater Sci 50(7):2914–2920
Zhu J, Cao G, Li Y et al (2019) Nd2O3 encapsulation-assisted surface passivation of Ni-rich LiNi0.8Co0.1Mn0.1O2 active material and its electrochemical performance. Electrochim Acta 325:134889
Li YC, Zhao WM, Xiang W et al (2018) Promoting the electrochemical performance of LiNi0.8Co0.1Mn0.1O2 cathode via LaAlO3 coating. J Alloys Compd 766:546–555
Sun G, Yin X, Yang W et al (2018) Synergistic effects of ion doping and surface-modifying for lithium transition-metal oxide: synthesis and characterization of La2O3-modified LiNi1/3Co1/3Mn1/3O2. Electrochim Acta 272:11–21
Liu J, Jiang X, Zhang Y et al (2018) Improvement of high-voltage electrochemical performance of surface modified LiNi0.6Co0.2Mn0.2O2 cathode by La2O3 coating. Int J Electrochem Sci 13:9816–9825
Ren T, Zhang J, Wang D et al (2018) Enhancing the high-voltage performances of Ni-rich cathode materials by homogeneous La2O3 coating via a freeze-drying assisted method. Ceram Int 44:14660–14666
Loghavi MM, Mohammadi-Manesh H, Eqra R (2019) Y2O3-decorated LiNi0.8Co0.15Al0.05O2 cathode material with improved electrochemical performance for lithium-ion batteries. J Electroanal Chem 848:113326
Guo S, Yuan B, Zhao H et al (2019) Dual-component LixTiO2 @silica functional coating in one layer for performance enhanced LiNi0.6Co0.2Mn0.2O2 cathode. Nano Energy 58:673–679
Wu Y, Li M, Wahyudi W, Sheng G, Miao X, Anthopoulos TD, Huang KW, Li Y, Lai Z (2019) Performance and stability improvement of layered NCM lithium-ion batteries at high voltage by a microporous Al2O3 sol-gel coating. ACS Omega 4(9):13972–13980
Kong JZ, Chen Y, Cao YQ et al (2019) Enhanced electrochemical performance of Ni-rich LiNi0.6Co0.2Mn0.2O2 coated by molecular layer deposition derived dual-functional C-Al2O3 composite coating. J Alloys Compd 799:89–98
Song JH, Bae J, Lee K et al (2018) Enhancement of high temperature cycling stability in high-nickel cathode materials with titanium doping. J Ind Eng Chem 68:124–128
Lv C, Yang J, Peng Y et al (2019) 1D Nb-doped LiNi1/3Co1/3Mn1/3O2 nanostructures as excellent cathodes for Li-ion battery. Electrochim Acta 297:258–266
Shang G, Tang Y, Lai Y et al (2019) Enhancing structural stability unto 4.5 V of Ni-rich cathodes by tungsten-doping for lithium storage. J Power Sources 423:246–254
Wang Z, Wang Z, Guo H et al (2015) Improving the cycling stability of LiCoO2 at 4.5 V through co-modification by Mg doping and zirconium oxyfluoride coating. Ceram Int 41:469–474
Meng K, Wang Z, Guo H, Li X (2017) Enhanced cycling stability of LiNi0.8Co0.1Mn0.1O2by reducing surface oxygen defects. Electrochim Acta 234:99–107
Kim JH, Park CW, Sun YK (2003) Synthesis and electrochemical behavior of Li[Li0.1Ni0.35-x/2CoxMn0.55-x/2]O2cathode materials. Solid State Ionics 164:43–49
Kim H, Kim MG, Jeong HY, Nam H, Cho J (2015) A new coating method for alleviating surface degradation of LiNi0.6Co0.2Mn0.2O2 cathode material: nanoscale surface treatment of primary particles. Nano Lett 15(3):2111–2119
Woo S-W, Myung S-T, Bang H et al (2009) Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al, mg) substitution. Electrochim Acta 54:3851–3856
Wang D, Li X, Wang Z et al (2016) Role of zirconium dopant on the structure and high voltage electrochemical performances of LiNi0.5Co0.2Mn0.3O2 cathode materials for lithium ion batteries. Electrochim Acta 188:48–56
Dastan D (2017) Effect of preparation methods on the properties of titania nanoparticles: solvothermal versus sol-gel. Appl Phys A Mater Sci Process 123:1–13
Jafari A, Alam MH, Dastan D et al (2019) Statistical, morphological, and corrosion behavior of PECVD derived cobalt oxide thin films. J Mater Sci Mater Electron 30:21185–21198
Dastan D, Chaure N, Kartha M (2017) Surfactants assisted solvothermal derived titania nanoparticles: synthesis and simulation. J Mater Sci Mater Electron 28:7784–7796
Wu N, Li X, Li JG et al (2018) Fabrication of Gd2O3-MgO nanocomposite optical ceramics with varied crystallographic modifications of Gd2O3 constituent. J Am Ceram Soc 101:4887–4891
Li G, Qi L, Xiao P et al (2018) Effect of precursor structures on the electrochemical performance of Ni-rich LiNi0.88Co0.12O2 cathode materials. Electrochim Acta 270:319–329
Wang C, Zhou F, Chen K et al (2015) Electrochemical properties of α-MoO3-coated Li [Li0.2Mn0. 54Ni0.13Co0.13]O2 cathode material for Li-ion batteries. Electrochim Acta 176:1171–1181
Chen H, Hu Q, Huang Z et al (2016) Synthesis and electrochemical study of Zr-doped Li[Li0.2Mn0.54Ni0.13Co0.13]O2 as cathode material for Li-ion battery. Ceram Int 42:263–269
Chen Q, Luo L, Wang L et al (2018) Enhanced electrochemical properties of Y2O3-coated-(lithium-manganese)-rich layered oxides as cathode materials for use in lithium-ion batteries. J Alloys Compd 735:1778–1786
Zuo H, Fu W, Fan R et al (2020) Bilayer carbon nanowires/nickel cobalt hydroxides nanostructures for high-performance supercapacitors. Mater Lett 263:127217
Yin XT, Dastan D, Wu FY, Li J (2019) Facile synthesis of SnO2/LaFeO3-XNX composite: photocatalytic activity and gas sensing performance. Nanomaterials 9(8):1163
Abbasi S, Hasanpour M, Ahmadpoor F et al (2019) Application of the statistical analysis methodology for photodegradation of methyl orange using a new nanocomposite containing modified TiO2 semiconductor with SnO2. Int J Environ Anal Chem 00:1–17
Dastan D, Banpurkar A (2017) Solution processable sol-gel derived titania gate dielectric for organic field effect transistors. J Mater Sci Mater Electron 28:3851–3859
Acknowledgments
This work is grateful for the funding of the Government of Chongzuo, Guangxi Zhuang Autonomous Region (No. 2019015) and Science and Technology Department of Guangxi Zhuang Autonomous Region, China (No. AD18281073).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 29 kb)
Rights and permissions
About this article
Cite this article
Li, Y., Chang, S., Zheng, J. et al. Dual-modification of Gd2O3 on the high-voltage electrochemical properties of LiNi0.8Co0.1Mn0.1O2 cathode materials via the solid-state method. J Solid State Electrochem 24, 863–872 (2020). https://doi.org/10.1007/s10008-020-04523-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-020-04523-3