Abstract
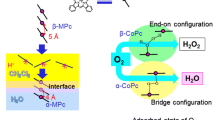
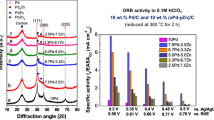
Catalytic activity of monometric metal macrocycles for oxygen reduction reaction (ORR) was investigated using rotating disk electrode voltammetry in acidic media. Iron(II) phthalocyanines (FePc) and cobalt(II) tetraphenylporphyrins (CoTPP) were immobilized on Au surfaces through molecular wires with different terminal groups of pyridine and isocyanide. The measured ORR behavior of FePc was largely influenced by the axial ligand while CoTPP promoted only the two-electron reaction regardless of the axial ligand. The FePc immobilized with pyridine was easily detached from the surface under the ORR condition, and was able to promote the four-electron reaction only under the high overpotential application. The FePc with isocyanide was more stable with the activity for the four-electron reaction, due to stronger electron donation to Fe central ions.






Similar content being viewed by others
References
Zagal JH, Bedioui F, Dodelet JP (2006) N4-macrocyclic metal complexes. Springer, Berlin
Tarasevich MR, Sadkowski A, Yeager E (1983) Comprehensive treatise of electrochemistry. In: Conway BE, Bockris JO, Yeager E, SUM K, White RE (eds) Vol. 7 Kinetics and Mechanisms of Electrode Processes, 1st edn. Plenum, NY
Zagal JH, Griveau S, Francisco Silva J, Nyokong T, Bedioui F (2010) Metallophthalocyanine-based molecular materials as catalysts for electrochemical reactions. Coord Chem Rev 254(23–24):2755–2791
McGuire R Jr, Dogutan DK, Teets TS, Suntivich J, Shao-Horn Y, Nocera DG (2010) Oxygen reduction reactivity of cobalt(II) hangman porphyrins. Chem Sci 1(3):411–414
Collman JP, Wagenknecht PS, Hutchison JE (1994) Molecular catalysts for multielectron redox reactions of small molecules: the “Cofacial Metallodiporphyrin” approach. Angew Chem Int Ed 33(15–16):1537–1554
Collman JP, Kim K (1986) Electrocatalytic four-electron reduction of dioxygen by iridium porphyrins adsorbed on graphite. J Am Chem Soc 108(24):7847–7849
Bytheway I, Hall MB (1994) Theoretical calculations of metal-dioxygen complexes. Chem Rev 94(3):639–658
Wei PJ, Yu GQ, Naruta Y, Liu JG (2014) Covalent grafting of carbon nanotubes with a biomimetic heme model compound to enhance oxygen reduction reactions. Angew Chem Int Ed 53(26):6659–6663
Cao R, Thapa R, Kim H, Xu X, Kim MG, Li Q, Park N, Liu M, Cho J (2013) Nat Commun 4:2076
Gewirth AA, Thorum MS (2010) Electroreduction of dioxygen for fuel-cell applications: materials and challenges. Inorg Chem 49(8):3557–3566
Zhang J (2008) PEM fuel cell electrocatalysts and catalyst layers: fundamentals and applications. Springer, Berlin
Banham D, Sa Y, Pei K, Ozaki J, Kishimoto T, Imashiro Y (2015) A review of the stability and durability of non-precious metal catalysts for the oxygen reduction reaction in proton exchange membrane fuel cells. J Power Sources 285:334–348
Ponce I, Francisco Silva J, Oñate R, Caroli Rezende M, Paez MA, Zagal JH, Pavez J, Mendizabal F, Miranda-Rojas S, Muñoz-Castro A, Arratia-Pérez R (2012) Enhancement of the catalytic activity of Fe phthalocyanine for the reduction of O2 anchored to au(111) via conjugated self-assembled monolayers of aromatic thiols as compared to cu phthalocyanine. J Phys Chem C 116(29):15329–15341
Morozan A, Campidelli S, Filoramo A, Jousselme B, Palacin S (2011) Catalytic activity of cobalt and iron phthalocyanines or porphyrins supported on different carbon nanotubes towards oxygen reduction reaction. Carbon 49(14):4839–4847
Sato S, Namba K, Hara K, Fukuoka A, Murakishi K, Uosaki K, Ikeda K (2016) Kinetic behavior of catalytic active sites connected with a conducting surface through various electronic coupling. J Phys Chem C 120(4):2159–2165
Sato S, Iwase S, Namba K, Ono T, Hara K, Fukuoka A, Uosaki K, Ikeda K (2018) Electrical matching at metal/molecule contacts for efficient heterogeneous charge transfer. ACS Nano 12(2):1228–1235
Sato S, Murakoshi K, Ikeda K (2016) Photoelectrochemical behavior of homo- and heterodimers of metalloporphyrins. Chem Lett 45(2):125–127
Tian ZQ, Ren B, Wu DY (2002) Surface-enhanced Raman scattering: from noble to transition metals and from rough surfaces to ordered nanostructures. J Phys Chem B 106(37):9463–9483
Inagaki M, Motobayashi K, Ikeda K (2017) Electrochemical THz-SERS observation of thiol monolayers on au(111) and (100) using nanoparticle-assisted gap-mode plasmon excitation. J Phys Chem Lett 8(17):4236–4240
Isvoranu C, Wang B, Schulte K, Ataman E, Knudsen J, Andersen JN, Bocquet ML, Schnadt J (2010) Tuning the spin state of iron phthalocyanine by ligand adsorption. J Phys Condens Matter 22:472002
Alsudairi A, Li J, Ramaswamy N, Mukerjee S, Abraham KM, Jia Q (2017) Resolving the iron phthalocyanine redox transitions for ORR catalysis in aqueous media. J Phys Chem Lett 8(13):2881–2886
Brown GM, Hopf FR, Meyer TJ, Whitten DG (1975) Effect of Extraplanar ligands on the redox properties and the site of oxidation in iron, ruthenium, and osmium porphyrin complexes. J Am Chem Soc 97(19):5385–5390
Funding
This research was partially supported by Grant-in-Aid for Challenging Research (Exploratory) (No. 18 K18999) from JSPS, and by program for Development of Environmental Technology using Nanotechnology from Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toyama, T., Sato, S., Motobayashi, K. et al. A rotating disk electrode study on catalytic activity of iron(II) phthalocyanine-modified electrodes for oxygen reduction in acidic media. J Solid State Electrochem 25, 141–147 (2021). https://doi.org/10.1007/s10008-019-04461-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04461-9