Abstract
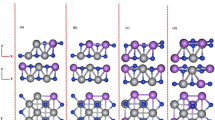
Graphite may store lithium or potassium, but not sodium, in its interlayer space under ambient conditions. It is, however, unclear whether binary alkali alloys of Li-Na, Li-K, and Na-K may substitute pure Li or K to form binary alkali alloy-graphite intercalation compounds. We investigate thermodynamics of the binary alloy-graphite intercalation compounds using density functional theory with van der Waals density functionals. We find Li-rich co-intercalation compounds and K-rich ones are associated with negative formation energies, and the Na-K alloy has the broadest domain of co-intercalation (approximately up to 36% Na). Because of convexity of the formation-energy functions, these compounds are metastable and tend to decompose even when formation energies are negative. Na metal is among the decomposition products. Binary Li-K alloys in graphite form segregated phases of LiC6 and KC8, and this allows one to fabricate Li-K mixed-ion batteries using graphite anodes, whereas Li-Na and Na-K alloys are thermodynamically unfavorable. The study highlights the importance of convexity of formation-energy functions in thermodynamics of alloy-graphite intercalation compounds.








Similar content being viewed by others
References
Lewis NS (2011) Powering the planet. MRS Bull 32:808–820
Bruce D, Haresh K, Jean-Marie T (2011) Electrical energy storage for the grid: a battery of choices. Science 334:928–935
Goodenough JB (2015) Energy storage materials: a perspective. Energy Storage Mater 1:158–161
Chen R, Luo R, Huang Y, Wu F, Li L (2016) Advanced high energy eensity secondary batteries with multi-electron reaction materials. Adv Sci 3(10):1600051
Manthiram A (2011) Materials challenges and opportunities of lithium ion batteries. J Phys Chem Lett 2(3):176–184
Goodenough JB, Park KS (2013) The Li-ion rechargeable battery: a perspective. J Am Chem Soc 135(4):1167–1176
Goodenough JB, Kim Y (2010) Challenges for rechargeable Li batteries. Chem Mater 22(3):587–603
Tarascon JM, Armand M (2001) Issues and challenges facing rechargeable lithium batteries. Nature 414(6861):359–367
Tarascon JM (2010) Is lithium the new gold? Nat Chem 2(6):510
Hong SY, Kim Y, Park Y, Choi A, Choi N-S, Lee KT (2013) Charge carriers in rechargeable batteries: Na ions vs. Li ions. Energy Environ Sci 6(7):2067
Liu Y, Artyukhov VI, Liu M, Harutyunyan AR, Yakobson BI (2013) Feasibility of lithium storage on graphene and its derivatives. J Phys Chem Lett 4(10):1737–1742
Xi X-T, Li W-H, Hou B-H, Yang Y, Gu Z-Y, Wu X-L (2019) Dendrite-free lithium anode enables the lithium//graphite dual-ion battery with much improved cyclic stability. ACS Appl Energy Mater 2(1):201–206
Li W-H, Ning Q-L, Xi X-T, Hou B-H, Guo J-Z, Yang Y, Chen B, Wu X-L (2019) Highly improved cycling stability of anion de-/intercalation in the graphite cathode for dual-ion batteries. Adv Mater 31(4):1804766
Wedepohl KH (1995) The composition of the continental Crust. Geochim Cosmochim Acta 59(7):1217–1232
Wang LP, Yu L, Wang X, Srinivasan M, Xu ZJ (2015) Recent developments in electrode materials for sodium-ion batteries. J Mater Chem A 3(18):9353–9378
Hwang JY, Myung ST, Sun YK (2017) Sodium-ion batteries: present and future. Chem Soc Rev 46(12):3529–3614
Wang Y-Y, Hou B-H, Guo J-Z, Ning Q-L, Pang W-L, Wang J, Lü C-L, Wu X-L (2018) An ultralong lifespan and low-temperature workable sodium-ion full battery for stationary energy storage. Adv Energy Mater 8(18):1703252
Pramudita JC, Sehrawat D, Goonetilleke D, Sharma N (2017) An initial review of the status of electrode materials for potassium-ion batteries. Adv Energy Mater 7(24):1602911
Yabuuchi N, Kubota K, Dahbi M, Komaba S (2014) Research development on sodium-ion batteries. Chem Rev 114(23):11636–11682
Slater MD, Kim D, Lee E, Johnson CS (2013) Sodium-ion batteries. Adv Funct Mater 23(8):947–958
Muralidharan N, Carter R, Oakes L, Cohn AP, Pint CL (2016) Strain engineering to modify the electrochemistry of energy storage electrodes. Sci Rep 6(1):27542
Wen Y, He K, Zhu Y, Han F, Xu Y, Matsuda I, Ishii Y, Cumings J, Wang C (2014) Expanded graphite as superior anode for sodium-ion batteries. Nat Commun 5(1):4033
Liu Y, Merinov BV, Goddard WA 3rd (2016) Origin of low sodium capacity in graphite and generally weak substrate binding of Na and Mg among alkali and alkaline earth metals. Proc Natl Acad Sci U S A 113(14):3735–3739
Moriwake H, Kuwabara A, Fisher CAJ, Ikuhara Y (2017) Why is sodium-intercalated graphite unstable? RSC Adv 7(58):36550–36554
Dresselhaus MS, Dresselhaus G (2002) Intercalation compounds of graphite. Adv Phys 51(1):1–186
Lücking F, Köser H, Jank M, Ritter A (1998) Iron powder, graphite and activated carbon as catalysts for the oxidation of 4-chlorophenol with hydrogen peroxide in aqueous solution. Water Res 32(9):2607–2614
Chambers A, Park C, Baker RM, Rodriguez N (1998) Hydrogen storage in graphite nanofibers. J Phys Chem B 102(22):4253–4256
Noel M, Santhanam R (1998) Electrochemistry of graphite intercalation compounds. J Power Sources 72(1):53–65
Sim HS, Kim TA, Lee KH, Park M (2012) Preparation of graphene nanosheets through repeated supercritical carbon dioxide process. Mater Lett 89:343–346
Knieke C, Berger A, Voigt M, Taylor RNK, Röhrl J, Peukert W (2010) Scalable production of graphene sheets by mechanical delamination. Carbon 48(11):3196–3204
Pruvost S, Hérold C, Hérold A, Lagrange P (2003) On the great difficulty of intercalating lithium with a second element into graphite. Carbon 41(6):1281–1289
Basu S, Zeller C, Flanders PJ, Fuerst CD, Johnson WD (1979) Synthesis and properties of lithium-graphite intercalation compounds. Mater Sci Eng 38(3):275–283
Antoine L, Gachon JC, Guerard D (1998) Intercalation of sodium-potassium alloys into graphite. MRS Proc 548:49
Iñiguez M, Alonso J (2000) Density functional-pseudopotential approach to the heat of formation in alloys of alkali metals. J Phys F Met Phys 11:2045
Kohn W, Sham LJ (1965) Self-consistent equations including exchange and correlation effects. Phys Rev 140(4A):A1133–A1138
Jones RO (2015) Density functional theory: its origins, rise to prominence, and future. Rev Mod Phys 87(3):897–923
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6(1):15–50
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B Condens Matter 50(24):17953–17979
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59(3):1758–1775
Klimeš J, Bowler DR, Michaelides A (2010) Chemical accuracy for the van der Waals density functional. J Phys Condens Matter 22(2):022201
Wang Z, Selbach SM, Grande T (2014) Van der Waals density functional study of the energetics of alkali metal intercalation in graphite. RSC Adv 4(8):3973–3983
Nobuhara K, Nakayama H, Nose M, Nakanishi S, Iba H (2013) First-principles study of alkali metal-graphite intercalation compounds. J Power Sources 243:585–587
Okamoto Y (2013) Density functional theory calculations of alkali metal (Li, Na, and K) graphite intercalation compounds. J Phys Chem C 118:16–19
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13(12):5188–5192
Klimes J, Bowler D, Michaelides A (2011) Van der Waals density functionals applied to solids. Phys Rev B 83(19):195131
Dion M, Rydberg H, SchröDer E, Langreth DC, Lundqvist BI (2004) Van der Waals density functional for general geometries. Phys Rev Lett 92(24):246401
Román-Pérez G, Soler JM (2009) Efficient implementation of a van der Waals density functional: application to double-wall carbon nanotubes. Phys Rev Lett 103(9):096102
Trucano P, Chen R (1975) Structure of graphite by neutron diffraction. Nature 258(5531):136–137
Guerard D, Herold A (1975) Intercalation of lithium into graphite and other carbons. Carbon 13(4):337–345
Nixon DE, Parry GS (1969) The expansion of the carbon-carbon bond length in potassium graphites. J Phys C Solid State Phys 2(10):1732–1741
Zhao J, Zou X, Zhu Y, Xu Y, Wang C (2016) Electrochemical intercalation of potassium into graphite. Adv Funct Mater 26(44):8103–8110
King HW (1966) Quantitative size-factors for metallic solid solutions. J Mater Sci 1(1):79–90
Herold A, Billaud D, Guerard D, Lagrange P (1977) Action compétitive de deux métaux Alcalins sur le graphite. Mater Sci Eng 31:25–28
Xue L, Gao H, Zhou W, Xin S, Park K, Li Y, Goodenough JB (2016) Liquid K-Na alloy anode enables dendrite-free potassium batteries. Adv Mater 28(43):9608–9612
Xu Z, Lv X, Chen J, Jiang L, Lai Y, Jie L (2016) Dispersion-corrected DFT investigation on defect chemistry and potassium migration in potassium-graphite intercalation compounds for potassium ion batteries anode materials. Carbon 107:885–894
Ye H, Zheng Z-J, Yao H-R, Liu S-C, Zuo T-T, Wu X-W, Yin Y-X, Li N-W, Gu J-J, Cao F-F, Guo Y-G (2019) Guiding uniform Li plating/stripping through lithium-aluminum alloying medium for long-life Li metal batteries. Angew Chem 131(4):1106–1111
Funding
This work is supported by Doctoral Fund of Ministry of Education of China (20133108120021), the National Natural Science Foundation of China for Youths (51302166), and Municipal Natural Science foundation of Shanghai. The computations are performed on Compmat cluster and Ziqiang4000 supercomputer of the High Performance Computing Center of Shanghai University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 942 kb)
Rights and permissions
About this article
Cite this article
Song, T., Xie, Y., Chen, Y. et al. Thermodynamics of graphite intercalation binary alloys of Li-Na, Na-K, and Li-K from van der Waals density functionals. J Solid State Electrochem 23, 2825–2834 (2019). https://doi.org/10.1007/s10008-019-04383-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04383-6