Abstract
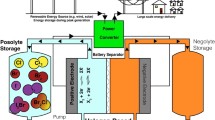
Hydrogen-bromate flow battery is a promising hybrid current source for air-deficient environment that functions by electrocatalyzed reactions of hydrogen oxidation and aqueous LiBrO3 reduction. The flow cell consists of porous carbonaceous cathode, platinum catalyzed hydrogen oxidation gas diffusion anode, and separating proton exchange membrane. Performance of the hydrogen-bromate flow battery single cell has been optimized by varying the catholyte feed rate, LiBrO3 concentration in catholyte, hydrogen pressure, membrane thickness, amount of porous carbon at cathode, and Pt loading at anode. Shape of the I-V curve is characterized by a sharp maximum of current, which indicates passivation of one of the electrodes. Combination of conventional reference electrode and home-made thin-film Luggin capillary has been used to monitor separately the polarizations of both flow cell electrodes. Poisoning of platinum hydrogen oxidation electrocatalyst by bromine species, which permeated the membrane, is shown as a major source of performance losses of hydrogen-bromate flow battery at high power density. Hypothesis supported by experiments claims that the degree of the platinum electrocatalyst poisoning is determined by the balance between the rates of the bromine species supply to anode and their removal by liquid water that permeates the membrane. Use of thinner proton exchange membrane and thinner carbonaceous cathode is a prerequisite to achieving high power density of the cell at high current efficiency of the cathode process. At 40 °C, area-specific power reaches 0.74 W cm−2 at the level of catholyte utilization equal to 0.93.

Graphical abstract








Similar content being viewed by others
References
Crompton TR (2000) Battery reference book, 3rd edn. Newnes, Oxford
Garche J, Dyer CK, Moseley PT, Ogumi Z, Rand DAJ, Scrosati B (eds) (2009) Encyclopedia of electrochemical power sources, 1st edn. – Newnes, 2013.
Daniel C (ed) (2011) Handbook of battery materials, 2nd edn. Wiley, Weinheim
Shen PK, Wang C-Y, Jiang SP, Sun X, Zhang J (eds) (2016) Electrochemical energy advanced materials and technologies. CRC press, Boca Raton
Aifantis E, Hackney SA, Kumar RV (eds) (2010) High energy density lithium batteries. Materials, engineering, applications. Wiley, Weinheim
Stolten D (ed) (2010) Hydrogen and fuel cells. Fundamentals, technologies and applications. Wiley, Weinheim
Kim KJ, Park MS, Kim YJ, Kim JH, Dou SX, Skyllas-Kazacos M (2015) A technology review of electrodes and reaction mechanisms in vanadium redox flow batteries. J Mater Chem A 3(33):16913–16933
Leung P, Shah AA, Sanz L, Flox C, Morante JR, Xu Q, Mohamed MR, Ponce de León C, Walsh FC (2017) Recent developments in organic redox flow batteries: a critical review. J Power Sources 360:243–283
Perrya ML, Weber AZ (2016) Advanced redox-flow batteries: a perspective. J Electrochem Soc 163(1):A5064–A5067
Noack J, Roznyatovskaya N, Herr T, Fischer P (2015) The chemistry of redox-flow batteries. Angew Chem Int Ed 54(34):9776–9809
Arenas LF, Ponce de León CP, Walsh FC (2017) Engineering aspects of the design, construction and performance of modular redox flow batteries for energy storage. J Energy Storage 11:119–153
Vorotyntsev MA, Tolmachev YV (2014) Fuel cells with chemically regenerative redox cathodes (review). Russ J Electrochem 50:403–411
Rajarathnam GP, Vassallo AM (2016) The zinc/bromine flow battery. Materials challenges and practical solutions for technology advancement. Springer, Sydney
Rubio-Garcia J, Kucernak A, Zhao D, Li D, Fahy K, Yufit V, Brandon N, Gomez-Gonzalez M (2019) Hydrogen/manganese hybrid redox flow battery. J Phys Energy 1:015006
Cho KT, Tucker MC, Weber AZ (2016) A review of hydrogen/halogen flow cells. Energy Technol 4(6):655–678
Zuo W, Li R, Zhou C, Li Y, Xia J, Liu J (2017) Battery-supercapacitor hybrid devices: recent progress and future prospects. Adv Sci 4:1600539
Tolmachev YV, Piatkivskyi A, Ryzhov VV, Konev DV, Vorotyntsev MA (2015) Energy cycle based on a high specific energy aqueous flow battery and its potential use for fully electric vehicles and for direct solar-to-chemical energy conversion. J Solid State Electrochem 19(9):2711–2722
Mussini T, Longhi P (1985) Bromine. In: Bard AJ, Parsons R, Jordan J (eds) Standard potentials in aqueous solutions. IUPAC, Marcel Dekker, New York
Bruno TJ, Lide DR (2015) In: Haynes WM (ed) CRC handbook of chemistry and physics, 97th edn. Boca Raton, CRC Press
Simmons JP, Waldeck WF (1931) The system lithium bromate-water. J Am Chem Soc 53(5):1725–1727
Mylius F, Funk R (1897) Ueber die Löslichkeit einiger leicht löslicher Salze in Wasser bei 18°. Ber Dtsch Chem Ges 30(2):1716–1725
Vorotyntsev MA, Antipov AE, Konev DV (2017) Bromate anion reduction: novel autocatalytic (EC″) mechanism of electrochemical processes. Its implication for redox flow batteries of high energy and power densities. Pure Appl Chem 89(10):1429–1448
Modestov AD, Konev DV, Tripachev OV, Antipov AE, Tolmachev YV, Vorotyntsev MA (2018) A hydrogen–bromate flow battery for air-deficient environments. Energy Technol 6(2):242–245
Bard AJ, Faulkner LR (eds) (2001) Electrochemical methods fundamentals and applications, vol ch. 12, second edn. Wiley, New York, pp 471–523
Vorotyntsev MA, Konev DV, Tolmachev YV (2015) Electroreduction of halogen oxoanions via autocatalytic redox mediation by halide anions: novel EC mechanism. Theory for stationary 1D regime. Electrochim Acta 173:779–795
Vorotyntsev MA, Antipov A (2016) Generalized Nernst layer model: application to bromate anion electroreduction and theory for the stationary 1D regime of proton transport limitations. ChemElectroChem 3:2227–2242
Vorotyntsev MA, Antipov AE (2018) Bromate electroreduction from acidic solution at rotating disc electrode. Theoretical study of the steady-state convective-diffusion transport for excess of bromate ions compared to protons. Electrochim Acta 261:113–126
Côrtes CES, Faria RB (2004) Kinetics and mechanism of bromate− bromide reaction catalyzed by acetate. Inorg Chem 43(4):1395–1402
Schmitz G (2007) Kinetics of the bromate–bromide reaction at high bromide concentrations. Int J Chem Kinet 39(1):17–21
Vorotyntsev MA, Antipov AE, Tolmachev YV, Antipov EM, Aldoshin SM (2016) Electroreduction of bromate anion in acidic solutions at the inactive rotating disc electrode under steady-state conditions: numerical modeling of the process with bromate anions being in excess compared to protons. Dokl Chem 468(1):141–147
Antipov AE, Vorotyntsev MA (2016) Electroreduction of bromate anion on inactive RDE under steady-state conditions: numerical study of ion transport processes and comproportionation reaction. Russ J Electrochem 52:925–932
Vorotyntsev MA, Antipov AE (2017) Bromate electroreduction from acidic solution at spherical microelectrode under steady-state conditions: theory for the redox-mediator autocatalytic (EC″) mechanism. Electrochim Acta 258:544–553
Modestov AD, Konev DV, Antipov AE, Petrov MM, Pichugov RD, Vorotyntsev MA (2018) Bromate electroreduction from sulfuric acid solution at rotating disk electrode: experimental study. Electrochim Acta 259:655–663
Konev DV, Antipov AE, Petrov MM, Shamraeva MA, Vorotyntsev MA (2018) Surprising dependence of the current density of bromate electroreduction on the microelectrode radius as manifestation of the autocatalytic redox-cycle (EC″) reaction mechanism. Electrochem Commun 86:76–79
Cho KT, Razaulla T (2019) Redox-mediated bromate based electrochemical energy system. J Electrochem Soc 166(2):A286–A296
Petrov MM, Konev DV, Kuznetsov VV, Antipov AE, Glazkov AT, Vorotyntsev MA (2019) Electrochemically driven evolution of Br-containing aqueous solution composition. J Electroanal Chem 836:125–133
Barna GG, Frank SN, Teherani TH, Weedon LD (1984) Lifetime studies in H2/Br2 fuel cells. J Electrochem Soc 131(9):1973–1980
Barna GG, Frank SN, Teherani TH (1982) Oxidation of H2 at gas diffusion electrodes in H2SO4 and HBr. J Electrochem Soc 129(11):2464–2468
Livshits V, Ulus A, Peled E (2006) High-power H2/Br2 fuel cell. Electrochem Commun 8:1358–1362
Cho KT, Tucker MC, Ding M, Ridgway P, Battaglia VS, Srinivasan V, Weber AZ (2015) Cyclic performance analysis of hydrogen/bromine flow batteries for grid-scale energy storage. ChemPlusChem 80(2):402–411
Tucker MC, Cho KT, Weber AZ, Lin G, Nguyen TV (2015) Optimization of electrode characteristics for the Br2/H2 redox flow cell. J Appl Electrochem 45:11–19
Goor-Dar M, Travitsky N, Peled E (2012) Study of hydrogen redox reactions on platinum nanoparticles in concentrated HBr solutions. J Power Sources 197:111–115
Nguyen TV, Kreutzer H, Yarlagadda V (2013) HER/HOR catalysts for the H2-Br2 fuel cell system. ECS Trans 53(7):75–81
Oh K, Weber AZ, Ju H (2017) Study of bromine species crossover in H2/Br2 redox flow batteries. Int J Hydrog Energy 42:3753–3766
Xu J, Scherson D (2013) Quantitative correlations between the normal incidence differential reflectance and the coverage of adsorbed bromide on a polycrystalline platinum rotating disk electrode. Anal Chem 85(5):2795–2801
Marković NM, Lucas CA, Gasteiger HA, Ross PN (1966) Bromide adsorption on Pt(100): rotating ring-Pt(100) disk electrode and surface X-ray scattering measurements. Surf Sci 365:229–240
Zalitis CM, Kramer D, Kucernak AR (2013) Electrocatalytic performance of fuel cell reactions at low catalyst loading and high mass transport. Phys Chem Chem Phys 15(12):4329–4340
Weber AZ, Newman J (2004) Modeling transport in polymer-electrolyte fuel cells. Chem Rev 104:4679–4726
Will FG (1979) Bromine diffusion through Nafion® perfluorinated ion exchange membranes. J Electrochem Soc 126(1):36–43
Li G, Jia Y, Zhang S, Li X, Li J, Li L (2017) The crossover behavior of bromine species in the metal-free flow battery. Appl Electrochem 47:261–272
Yeo RS, McBreen J (1682) Transport properties of Nafion membranes in electrochemically regenerative hydrogen/halogen cells. J Electrochem Soc 126:1682–1687
Park JW, Wycisk R, Pintauro PN (2015) Nafion/PVDF nanofiber composite membranes for regenerative hydrogen/bromine fuel cells. J Membr Sci 490:103–112
Chen Q, Gerhardt MR, Hartle L, Aziz MJ (2016) A quinone-bromide flow battery with 1 W/cm2 power density. J Electrochem Soc 163(1):A5010–A5013
Gerhardt MR, Tong L, Gómez-Bombarelli R, Chen Q, Marshak MP, Galvin CJ, Aspuru-Guzik A, Gordon RG, Aziz MJ (2017) Anthraquinone derivatives in aqueous flow batteries. Adv Energy Mater 7(8):1601488
Cho KT, Albertus P, Battaglia V, Kojic A, Srinivasan V, Weber AZ (2013) Optimization and analysis of high-power hydrogen/bromine-flow batteries for grid-scale energy storage. Energy Technol 1:596–608
Tucker MC, Cho KT, Spingler FB, Weber AZ, Lin G (2015) Impact of membrane characteristics on the performance and cycling of the Br2–H2 redox flow cell. J Power Sources 284:212–221
Park JW, Wycisk R, Lin G, Chong PY, Powers D, Van Nguyen T, Dowd RP, Pintauro PN (2017) Electrospun Nafion/PVDF single-fiber blended membranes for regenerative H2/Br2 fuel cells. J Membr Sci 541:85–92
Darling RM, Weber AZ, Tucker MC, Perry ML (2016) The influence of electric field on crossover in redox-flow batteries. J Electrochem Soc 163(1):A5014–A5022
Wiberg N (ed) (2001) Holleman-Wiberg’s inorganic chemistry. Academic Press, New York
Kusoglu A, Cho KT, Prato RA, Weber AZ (2013) Structural and transport properties of Nafion in hydrobromic-acid solutions. Solid State Ionics 252:68–74
Podgorsek A, Stavber S, Zupan M, Iskra J, Padua AAH, Gomes MFC (2008) Solvation of halogens in fluorous phases. Experimental and simulation data for F2, Cl2, and Br2 in several fluorinated liquids. J Phys Chem B 112(21):6653–6664
Van Zee NJ, Dragojlovic V (2009) Phase-vanishing reactions with PTFE (Teflon) as a phase screen. Org Lett 11:3190–3193
Ding C, Zhang H, Li X, Liu T, Xing F (2013) Vanadium flow battery for energy storage: prospects and challenges. J Phys Chem Lett 4(8):1281–1294
Roe S, Menictas C, Skyllas-Kazacos M (2016) A high energy density vanadium redox flow battery with 3 M vanadium electrolyte. J Electrochem Soc 163(1):A5023–A5028
Kear G, Shah AA, Walsh FC (2012) Development of the all-vanadium redox flow battery for energy storage: a review of technological, financial and policy aspects. Int J Energy Res 36(11):1105–1120
Skyllas-Kazacos M, Chakrabarti MH, Hajimolana SA, Mjalli FS, Saleem M (2011) Progress in flow battery research and development. J Electrochem Soc 158(8):R55–R79
Weber AZ, Mench MM, Meyers JP, Ross PN, Gostick JT, Liu Q (2011) Redox flow batteries: a review. J Appl Electrochem 41:1137
Patnaik P (2003) Handbook of inorganic chemicals. McGraw-Hill, New York
Griffith RO, McKeown A, Winn AG (1932) The bromine-bromide-tribromide equilibrium. Trans Faraday Soc 28:101–107
Wu YC, Feng D (1995) The second dissociation constant of sulfuric acid at various temperatures by the conductometric method. J Solut Chem 24(2):133–144
Funding
This research was financially supported by the Russian Ministry of Education and Research (Grant № 14.574.21.0150, UIN RFMEFI57417X0150).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Bromate utilization in flow cell can reach 100% in single pass.
• Hydrogen electrooxidation is an important contributor to performance losses.
• Facile water transport through thin membrane mitigates anode poisoning.
• 93% conversion of BrO3- to Br- at 1.05 A cm-2 and 0.74 W cm-2 has been achieved.
Rights and permissions
About this article
Cite this article
Modestov, A.D., Konev, D.V., Antipov, A.E. et al. Hydrogen-bromate flow battery: can one reach both high bromate utilization and specific power?. J Solid State Electrochem 23, 3075–3088 (2019). https://doi.org/10.1007/s10008-019-04371-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04371-w