Abstract
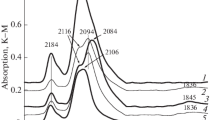
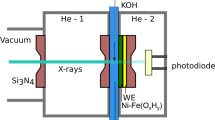
In situ X-ray absorption spectroscopy (XAS) was applied to investigate the Sn adlayer on platinized (pl-) Pt electrode in deaerated 0.2 M HClO4 solution containing 10−3 M Sn2+ in relation to the effect of Sn addition on electrocatalysis of Pt. A periodical emersion method under potentiostatic polarization, using pl-Pt plate (roughness factor Sr = 770) as a working electrode was employed to detect sensitively the sub-monolayer coverage of Sn on Pt. The Sn K-edge absorption spectra in a scanning XAS mode were measured by monitoring the Sn Kα1 fluorescence line. The Sn K-edge absorption near-edge structure (XANES) has indicated that the Sn species adsorbed on the pl-Pt electrode is partly oxygenated in the Sn-underpotential (UPD) region between − 0.05 and 0.25 V (RHE) which is overlapped with the UPD region of hydrogen. The extended X-ray absorption fine structure (EXAFS) analysis has supported a Sn overlayer model in which Sn atom occupies the hollow site of the nearest neighbor Pt atoms and is further bound with oxygen atoms in the Sn-UPD region. The coordination number of the Sn–Pt bond or Sn–Sn bond decreases with increasing potential, while the coordination number of the Sn–O bond increases reversely. In the potential region between 0.45 and 0.85 V (RHE), the EXAFS analysis has suggested that two-dimensional surface Sn oxide forms on the pl-Pt electrode, which is supported from the potential-pH equilibrium diagram of the Sn/H2O system.













Similar content being viewed by others
References
Motoo S, Shibata M, Watanabe M (1980) J Electroanal Chem 110(1-3):103–109
Gasteiger HA, Markovic NM, Ross PN (1995) J Phys Chem 99(22):8945–8949
Antolini E, Gonzalez ER (2010) Electrochim Acta 56(1):1–14
Stevanovíc S, Tripkovíc D, Tripkovíc V, Miníc D, Gavrilovíc A, Tripkvíc A, Jovanovíc VM (2014) J Phys Chem C 118(1):278–289
Motoo S, Watanabe M (1976) J Electroanal Chem 69(3):429–413
Watanabe M, Furuuchi Y, Motoo S (1985) J Electroanal Chem 191(2):367–375
Vassiliev YB, Bagotzky VS, Osetrova NV, Mikkailova AA (1979) J Electroanal Chem 97(1):63–76
Sobkowski J, Franaszczuk K, Piasecki A (1985) J Electroanal Chem 196(1):145–156
Wei ZD, Li LL, Luo YH, Yan C, Sun CX, Yin GZ, Shen PK (2006) J Phys Chem B 110(51):26055–26061
Beden B, Kadirgan F, Lamy C, Leger JM (1981) J Electroanal Chem 127(1-3):75–85
Haner AN, Ross PN (1991) J Phys Chem 95(9):3740–3746
Campbell SA, Parson R (1992) J Chem Soc Faraday Trans 88(6):833–841
Lamy-Pitara E, Quazzani-Benhima EE, Barbier J, Cahoreau M, Caisso J (1994) J Electroanal Chem 372(1-2):233–242
Janssen MMP, Moolhuysen J (1976) Electrochim Acta 21(11):861–868
Janssen MMP, Moolhuysen J (1977) J Catal 46(3):289–296
Wang K, Gasteiger HA, Markovic NM, Ross PN (1996) Electrochim Acta 41(16):2587–2593
Stamenkovic V, Aren M, Blizanac BB, Mayrhofer KJJ, Ross PN, Markovic NM (2005) Surf Sci 576(1-3):145–157
Liu Z, Reed D, Kwon G, Shamsuzzoha M, Nikles DE (2007) J Phys Chem C 111(38):14223–14229
Cathro KJ (1969) J Electrochem Soc 116(11):1608–1611
Katayama A (1980) J Phys Chem 84(4):376–381
Schryer DR, Upchurch BT, Van Norman JD, Brown KG, Schryer J (1990) J Catal 122(1):193–197
Aramata A, Toyoshima I, Enyo M (1992) Electrochim Acta 37(8):1317–1320
Grass K, Lintz H-G (1997) J Catal 172(2):446–452
Abruna HD (1991) In: Abruna HD (ed), Modern techniques for in-situ interface charctrerization Electrochemical interfaces chapt 1. Modern techniques for in-situ interface charctrerization. VCH Pub Inc, New York
Bunker G (2010) Introduction to XAFS. A practical guide to X-ray absorption fine structure spectroscopy. Cambridge University Press, Cambridge
Nagy Z (2011) J Solid State Electrochem 15(7-8):1679–1695
Russell AE, Rose A (2004) Chem Rev 104(10):4613–4635
Meitzner G, Via GH, Lytle FW, Fung SC, Sinfelt JH (1988) J Phys Chem 92(10):2925–2932
Borgna A, Stagg SM, Resasco DE (1998) J Phys Chem B 102(26):5077–5081
Pinxt HHCM, Kuster BFM, Koningsberger DC, Martin GB (1998) Catal Today 39(4):351–361
Mukerjee S, McBreen J (1999) J Electrochem Soc 146(2):600–606
Roman-Martinez MC, Cazorla-Amoros D, Yamashita H, de Miguel S, Scelza OA (2000) Langmuir 16(3):1123–1131
Ramallo-Lopez JM, Santori GF, Giovanetti L, Casella ML, Ferretti OA, Requejo FG (2003) J Phys Chem B 107(41):11441–11451
Melke J, Schoekel A, Dixon D, Cremers C, Ramaker DE, Roth C (2010) J Phys Chem C 114(13):5914–5925
Inoue T, Tomishige K, Iwasawa Y (1996) J Chem Soc Faraday Trans 92(3):461–467
Hansen WN, Wang CL, Humpherys TW (1978) J Electroanal Chem 93(2):87–98
Kolb DM, Hansen WN (1979) Surf Sci 79(1):205–211
Stefan IC, Scherson DA (2003) J Electroanal Chem 554-555:361–366
Samant MG, Borges GL, Gordon JG II, Melroy OR, Blum L (1987) J Am Chem Soc 109(20):5970–5974
Melroy OR, Samant MG, Borges GL, Gordon JG II, Blum L, White JH, Albarelli MJ, MicMillan M, Abruna HD (1988) Langmuir 4(3):728–732
Tadjeddine A, Tourillon G, Guay D (1991) Electrochim Acta 36(11-12):1859–1862
Tadjeddine A, Lahrichi A, Tourillon G (1993) J Electroanal Chem 360(1-2):261–270
Soldo Y, Sibert E, Tourillon G, Hazemann JL, Lévy JP, Aberdam D, Faure R, Durand R (2002) Electrochim Acta 47(19):3081–3091
Durand R, Faure R, Aberdam D, Salem C, Tourillon G, Guay D, Ladouceur M (1992) Electrochim Acta 37(11):1977–1982
Lee JRI, O’Malley RL, O’Connell TJ, Vollmer A, Rayment T (2010) Electrochim Acta 55(28):8532–8538
Seo M, Fushimi K, Aoki Y, Habazaki H, Inaba M, Yokomizo M, Hayakawa T, Nakayama T (2012) J Electroanal Chem 671:7–15
Seo M, Habazaki H, Inaba M, Yokomizo M, Wakabayashi T, Nakayama T (2014) J Electrochem Soc 161(4):H195–H202
Feltham AM, Spiro M (1971) Chem Rev 71(2):177–193
Seo M, Hyono A, Habazaki H, Nakayama T (2014) J Electrochem Soc 161(12):C550–C556
Du K, Ernst F, Pelsozy MC, Barthel J, Tillmann K (2010) Acta Mater 58(3):836–845
Gilman S (1964) Electrochim Acta 9(7):1025–1046
Brummer SB (1965) J Phys Chem 69(2):562–571
Brummer SB, Ford JI, Turner MJ (1965) J Phys Chem 69(10):3424–3433
Woods R (1968) Electrochim Acta 13(10):1967–1972
Biegler T (1969) J Electrochem Soc 116(8):1131–1137
Kolb DM, Przasnyski M, Gerischer H (1974) J Electroanal Chem 54(1):25–38
Kolb DM (1978) In: Gerischer H, Tobias CW (eds) Advances in electrochemistry and electrochemical engineering vol. Wiley, New York, p 1
Koma A, Yagi K, Tsukada AM (1987) Handbook of surface physics technology. Maruzen, Tokyo
Herrero E, Buller LJ, Abruna HD (2001) Chem Rev 101(7):1897–1930
Ankudinov AL, Ravel B, Rehr JJ, Condrason SD (1998) Phys Rev B 58(12):7565–7576
Ankudinov AL, Bouldin CE, Rehr JJ, Sim J, Hung H (2002) Phys Rev B 61:104107–104117
Ku Y, Overbury SH (1992) Surf Sci 373:341–352
Woodruff DP, Vlieg E (2002) In: Woodruff DP (ed) The chemical physics of solid surfaces vol 10. Surface alloys and alloy surfaces,chapt 8. Elsevier Science, Amsterdam
Overbury SH, Ku Y (1992) Phys Rev B 46(12):7868–7872
Brown D, Quinn PD, Woodruff DP, Bailey P, Noakes CQ (2000) Phys Rev B 61(11):7706–7715
Overbury SH, Mullins DR, Paffett MT, Koel BE (1991) Surf Sci 254(1-3):45–57
Li YD, Koel BE (1995) Surf Sci 330(2):193–206
Woodruff DP, Robinson J (2003) Appl Surf Sci 219(1-2):1–10
Yoshitake H, Yamazaki O, Ota K (1994) J Electrochem Soc 141(9):2516–2521
Klimenkov M, Nepijko S, Kuhlenbeck H, Bäumer M, Schlögl R, Freund H-L (1997) Surf Sci 391(1-3):27–36
Ankudinov AL, Rehr JJ, Low JJ, Bare SR (2002) J Chem Phys 116(5):1911–1919
Clausen BS, Grabek L, Topse H, Hansen LB, Stoltze P, Norskov JK, Nielsen OH (1993) J Catal 141(2):368–379
Koningsberger DC, Mojet BL, Van Dorssen GE, Ramaker DE (2003) Top Catal 10:143–155
Monti F (1996) J Synchrotron Radiat 3(3):129–195
Clausen BS, Norskov JK (2000) Top Catal 10(3/4):221–230
Bus E, Miller JT, Kropf AJ, Prins R, van Bokhoven JA (2006) Phys Chem Chem Phys 8(27):3248–32358
Sanson A (2009) J Synchrotron Radiat 16(6):864–868
Dalba G, Fornasini P, Rocca F (1993) Phys Rev B 47:8501–8514
Nakamura M, Imai R, Otsuka N, Hoshi N, Sakata O (2013) J Phys Chem C 117(35):18139–18143
Pourbaix M (1979) Atlas of electrochemical equilibria in aqueous solutions. NACE, Houston, pp 475–484
Acknowledgments
The XAS measurements were performed at the BL16B2 beam line of SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute (JASRI) (Proposal Nos. 2012A5320, 2012B5320).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Seo, M., Habazaki, H., Inaba, M. et al. In situ X-ray absorption spectroscopy of Sn species adsorbed on platinized platinum electrode in perchloric acid solution containing stannous ions. J Solid State Electrochem 23, 2261–2275 (2019). https://doi.org/10.1007/s10008-019-04326-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04326-1