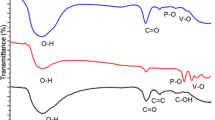
Abstract
Graphene oxide–modified poly (vinyl alcohol)/sodium sulfate-sodium molybdate (GO/PVA-Na2SO4-Na2MoO4, GPSS) gel polymer electrolyte and nickel molybdate (NiMoO4) electrode are integrated to fabricate carbon paper (CP) supercapacitor to improve capacitance performance. GO in PVA gel can introduce an effective ion transport pathway to improve ionic conductivity of gel polymer electrolyte. The ionic conductivity increases from 3.73 mS cm−1 for PVA-Na2SO4 to 6.46 mS cm−1 for GO/PVA-Na2SO4 at optimal GO mass ratio of 0.6% in GO/PVA gel. It also obviously increases from 4.33 mS cm−1 for PVA-Na2SO4-Na2MoO4 to 28.86 mS cm−1 for GO/PVA-Na2SO4-Na2MoO4. Both Na2MoO4 electrolyte and NiMoO4 electrode show reversible redox electroactivity to provide superior pseudocapacitance. Accordingly, the CP supercapacitor using GPSS gel shows specific capacitance of 41.67 mF cm−2 and energy density of 70.02 mWh m−2 at 0.5 mA cm−2, presenting higher performance than 15.91 mF cm−2 and 26.74 mWh m−2 using GO/PVA-Na2SO4 gel. Furthermore, the NiMoO4/CP supercapacitor using GPSS gel shows even higher specific capacitance of 78.18 mF cm−2 and energy density of 131.39 mWh m−2 at 0.5 mA cm−2. It also exhibits high cycling capacitance retention of 85% at 0.5 mA cm−2 for 1000 cycles. The improved capacitance performance of CP supercapacitor using Na2MoO4 gel polymer electrolyte and NiMoO4 electrode is ascribed to reversible redox reaction of Mo(VI)/Mo(V), Mo(VI)/Mo(IV), and Ni(II)/Ni(III). The NiMoO4/CP supercapacitor using GO/Na2MoO4 gel polymer electrolyte becomes desirable for the promising application in energy storage devices.











Similar content being viewed by others
References
Lu X, Yu M, Wang G, Tong Y, Li Y (2014) Flexible solid-state supercapacitors: design, fabrication and applications. Energy Environ Sci 7:2160–2181
Xie Y (2019) Electrochemical performance of transition metal-coordinated polypyrrole: A Mini Review. Chem Rec 19:1–16
Zhao Z, Xie Y, Lu L (2018) Electrochemical performance of polyaniline-derivated nitrogen-doped carbon nanowires. Electrochim Acta 283:1618–1631
Zhao Z, Xie Y (2018) Electrochemical supercapacitor performance of boron and nitrogen co-doped porous carbon nanowires. J Power Sources 400:264–276
Zhou Y, Xie Y (2018) Enhanced electrochemical stability of carbon quantum dots-incorporated and ferrous-coordinated polypyrrole for supercapacitor. J Solid State Electrochem 22:2515–2529
Xie Y, Zhou Y (2018) Enhanced electrochemical stability of CuCo bimetallic-coordinated polypyrrole. Electrochim Acta 290:419–428
Xie Y, Sha X (2018) Electrochemical cycling stability of nickel (II) coordinated polyaniline. Synth Met 237:29–39
Lu L, Xie Y (2019) Phosphomolybdic acid cluster bridging carbon dots and polyaniline nanofibers for effective electrochemical energy storage. J Mater Sci 54:4842–4858
Xie Y, Sun P (2018) Electrochemical performance of interspace-expanded molybdenum disulfide few-layer. J Nanopart Res 20:183
Eskusson J, Rauwel P, Nerut J, Janes A (2016) A Hybrid Capacitor Based on Fe3O4-Graphene Nanocomposite/Few-Layer Graphene in Different Aqueous Electrolytes. J Electrochem Soc 163:A2768–A2775
Hu S, Ribeiro EL, Davari SA, Tian M, Mukherjee D, Khomami B (2017) Hybrid nanocomposites of nanostructured Co3O4 interfaced with reduced/nitrogen-doped graphene oxides for selective improvements in electrocatalytic and/or supercapacitive properties. RSC Adv 7:33166–33176
Li Z, Zhang W, Liu Y, Guo J, Yang B (2018) 2D nickel oxide nanosheets with highly porous structure for high performance capacitive energy storage. J Phys D: Appl Phys 51:045302
Gao Z, Yang WL, Wang J, Song NN, Li XD (2015) Flexible all-solid-state hierarchical NiCo2O4/porous graphene paper asymmetric supercapacitors with an exceptional combination of electrochemical properties. Nano Energy 13:306–317
Chen YP, Liu BR, Liu Q, Wang J, Li ZS, Jing XY, Liu LH (2015) Coaxial CoMoO4 nanowire arrays with chemically integrated conductive coating for high-performance flexible all-solid-state asymmetric supercapacitors. Nanoscale 7:15159–15167
Watcharatharapong T, Sundaram MM, Chakraborty S, Li D, Shafiullah G, Aughterson RD, Ahuja R (2017) Effect of Transition Metal Cations on Stability Enhancement for Molybdate-Based Hybrid Supercapacitor. Acs Appl Mater Interface 9:17977–17991
Lu L, Xie Y, Zhao Z (2018) Improved electrochemical stability of NixCo2x(OH)(6x)/NiCo2O4 electrode material. J Alloys Compd 731:903–913
Kumar Y, Pandey GP, Hashmi SA (2012) Gel Polymer Electrolyte Based Electrical Double Layer Capacitors: Comparative Study with Multiwalled Carbon Nanotubes and Activated Carbon Electrodes. J Phys Chem C 116:26118–26127
Fernicola A, Weise FC, Greenbaum SG, Kagimoto J, Scrosati B, Soleto A (2009) Lithium-Ion-Conducting Electrolytes: From an Ionic Liquid to the Polymer Membrane. J Electrochem Soc 156:A514–A520
Wang P, Zakeeruddin SM, Moser JE, Nazeeruddin MK, Sekiguchi T, Gratzel M (2003) A stable quasi-solid-state dye-sensitized solar cell with an amphiphilic ruthenium sensitizer and polymer gel electrolyte. Nat Mater 2:402–407
Verma P, Maire P, Novak P (2010) A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries. Electrochim Acta 55:6332–6341
Hashmi SA, Latham RJ, Linford RG, Schlindwein WS (1998) Conducting polymer-based electrochemical redox supercapacitors using proton and lithium ion conducting polymer electrolytes. Polym Int 47:28–33
Meng C, Liu C, Chen L, Hu C, Fan S (2010) Highly flexible and all-solid-state paperlike polymer supercapacitors. Nano Lett 10:4025–4031
Yang CC, Hsu ST, Chien WC (2005) All solid-state electric double-layer capacitors based on alkaline polyvinyl alcohol polymer electrolytes. J Power Sources 152:303–310
Yang C-C, Wu GM (2009) Study of microporous PVA/PVC composite polymer membrane and it application to MnO2 capacitors. Mater Chem Phys 114:948–955
Lewandowski A, Zajder M, Frackowiak E, Beguin F (2001) Supercapacitor based on activated carbon and polyethylene oxide-KOH-H2O polymer electrolyte. Electrochim Acta 46:2777–2780
Lee KT, Wu NL (2008) Manganese oxide electrochemical capacitor with potassium poly(acrylate) hydrogel electrolyte. J Power Sources 179:430–434
Xie Y, Wang J (2018) Capacitance performance of carbon paper supercapacitor using redox-mediated gel polymer electrolyte. J Sol-Gel Sci Technol 86:760–772
Yu H, Wu J, Fan L, Xu K, Zhong X, Lin Y, Lin J (2011) Improvement of the performance for quasi-solid-state supercapacitor by using PVA–KOH–KI polymer gel electrolyte. Electrochim Acta 56:6881–6886
Ma G, Li J, Sun K, Peng H, Mu J, Lei Z (2014) High performance solid-state supercapacitor with PVA–KOH–K3[Fe(CN)6] gel polymer as electrolyte and separator. J Power Sources 256:281–287
Veerasubramani GK, Krishnamoorthy K, Pazhamalai P, Kim SJ (2016) Enhanced electrochemical performances of graphene based solid-state flexible cable type supercapacitor using redox mediated polymer gel electrolyte. Carbon 105:638–648
Xu D, Hu W, Sun XN, Cui P, Chen XY (2017) Redox additives of Na 2 MoO 4 and KI: Synergistic effect and the improved capacitive performances for carbon-based supercapacitors. J Power Sources 341:448–456
Senthilkumar ST, Selvan RK, Melo JS, Sanjeeviraja C (2013) High Performance Solid-State Electric Double Layer Capacitor from Redox Mediated Gel Polymer Electrolyte and Renewable Tamarind Fruit Shell Derived Porous Carbon. Acs Appl Mater Interface 5:10541–10550
Senthilkumar ST, Selvan ARK, Ponpandian AN, Meloc BJS, Leed YS (2013) Improved performance of electric double layer capacitor using redox additive (VO2+/VO2+) aqueous electrolyte. J Mater Chem A 1:7913–7919
Yu H, Wu J, Fan L, Lin Y, Xu K, Tang Z, Cheng C, Tang S, Lin J, Huang M, Lan Z (2012) A novel redox-mediated gel polymer electrolyte for high-performance supercapacitor. J Power Sources 198:402–407
Ma G, Dong M, Sun K, Feng E, Peng H, Lei Z (2015) A redox mediator doped gel polymer as an electrolyte and separator for a high performance solid state supercapacitor. J Mater Chem A 3:4035–4041
Pan S, Deng J, Guan G, Zhang Y, Chen P, Ren J, Peng H (2015) A redox-active gel electrolyte for fiber-shaped supercapacitor with high area specific capacitance. J Mater Chem A 3:6286–6290
Sun K, Ran F, Zhao G, Zhu Y, Zheng Y, Ma M, Zheng X, Ma G, Lei Z (2016) High energy density of quasi-solid-state supercapacitor based on redox-mediated gel polymer electrolyte. RSC Adv 6:55225–55232
Huang Y-F, Wu P-F, Zhang M-Q, Ruan W-H, Giannelis EP (2014) Boron cross-linked graphene oxide/polyvinyl alcohol nanocomposite gel electrolyte for flexible solid-state electric double layer capacitor with high performance. Electrochim Acta 132:103–111
Yang X, Zhang F, Zhang L, Zhang T, Huang Y, Chen Y (2013) A High-Performance Graphene Oxide-Doped Ion Gel as Gel Polymer Electrolyte for All-Solid-State Supercapacitor Applications. Adv Funct Mater 23:3353–3360
Ni Z, Wang Y, Yu T, Shen Z (2008) Raman spectroscopy and imaging of graphene. Nano Res 1:273–291
Jothi PR, Kannan S, Velayutham G (2015) Enhanced methanol electro-oxidation over in-situ carbon and graphene supported one dimensional NiMoO4 nanorods. J Power Sources 277:350–359
Niyogi S, Bekyarova E, Itkis ME, Zhang H, Shepperd K, Hicks J, Sprinkle M, Berger C, Lau CN, deHeer WA, Conrad EH, Haddon RC (2010) Spectroscopy of Covalently Functionalized Graphene. Nano Lett 10:4061–4066
Su CY, Xu YP, Zhang WJ, Zhao JW, Tang XH, Tsai CH, Li LJ (2009) Electrical and Spectroscopic Characterizations of Ultra-Large Reduced Graphene Oxide Monolayers. Chem Mater 21:5674–5680
Reddy BJ, Vickraman P, Justin AS (2019) Synthesis and Characterization of Graphene/Binary Metal Molybdate (Graphene/Zn1-xNixMoO4) Nanocomposite for Supercapacitors. Phys Status Solidi A 216:1800595
Ye Y-S, Cheng M-Y, Xie X-L, Rick J, Huang Y-J, Chang F-C, Hwang B-J (2013) Alkali doped polyvinyl alcohol/graphene electrolyte for direct methanol alkaline fuel cells. J Power Sources 239:424–432
Cao Y-C, Xu C, Wu X, Wang X, Xing L, Scott K (2011) A poly (ethylene oxide)/graphene oxide electrolyte membrane for low temperature polymer fuel cells. J Power Sources 196:8377–8382
Li WS, Tian LP, Huang QM, Li H, Chen HY, Lian XP (2002) Catalytic oxidation of methanol on molybdate-modified platinum electrode in sulfuric acid solution. J Power Sources 104:281–288
Moutarlier V, Gigandet MP, Pagetti J, Ricq L (2003) Molybdate/sulfuric acid anodising of 2024-aluminium alloy: influence of inhibitor concentration on film growth and on corrosion resistance. Surface and Coatings Technology 173:87–95
Jothi PR, Shanthi K, Salunkhe RR, Pramanik M, Malgras V, Alshehri SM, Yamauchi Y (2015) Synthesis and Characterization of α-NiMoO4 Nanorods for Supercapacitor Application. Eur J Inorg Chem (22):3694–3699
Ramkumar R, Sundaram MM (2016) Electrochemical synthesis of polyaniline crosslinked NiMoO4 nanofibre dendrites for energy storage devices. New J Chem 40:7456–7464
Moreno B, Chinarro E, Colomer MT, Jurado JR (2010) Combustion Synthesis and Electrical Behavior of Nanometric β-NiMoO4. J Phys Chem C 114:4251–4257
Li P, Ruan C, Xu J, Xie Y (2019) Enhanced capacitive performance of CoO-modified NiMoO4 nanohybrid as advanced electrodes for asymmetric supercapacitor. J Alloys Compd 791:152–165
Sun K, Feng E, Peng H, Ma G, Wu Y, Wang H, Lei Z (2015) A simple and high-performance supercapacitor based on nitrogen-doped porous carbon in redox-mediated sodium molybdate electrolyte. Electrochim Acta 158:361–367
Yang X, Zhang L, Zhang F, Zhang T, Huang Y, Chen Y (2014) A high-performance all-solid-state supercapacitor with graphene-doped carbon material electrodes and a graphene oxide-doped ion gel electrolyte. Carbon 72:381–386
Verma ML, Minakshi M, Singh NK (2014) Synthesis and Characterization of Solid Polymer Electrolyte based on Activated Carbon for Solid State Capacitor. Electrochim Acta 137:497–503
Minakshi M, Singh P, Sharma N, Backford M, Ionescu M (2011) Lithium Extraction-Insertion from/into LiCoPO4 in Aqueous Batteries. Ind Eng Chem Res 50:1899–1905
Verma ML, Minakshi M, Singh NK (2014) Structural and Electrochemical Properties of Nanocomposite Polymer Electrolyte for Electrochemical Devices. Ind Eng Chem Res 53:14993–15001
Funding
The work was financially supported by the National Natural Science Foundation of China (no. 21373047), Graduate Innovation Program of Jiangsu Province (KYCX18_0080), the Fundamental Research Funds for the Central Universities (2242018K41024), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xie, Y., Zhang, Y. Electrochemical performance of carbon paper supercapacitor using sodium molybdate gel polymer electrolyte and nickel molybdate electrode. J Solid State Electrochem 23, 1911–1927 (2019). https://doi.org/10.1007/s10008-019-04260-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-019-04260-2