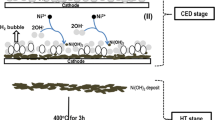
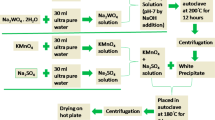
Abstract
A manganese oxide (MnO2) nanoplate-type electrode has been prepared using galvanostatic electrodeposition method with an aqueous manganese sulfate solution and characterized for its structural, morphological, compositional, and surface wettability studies and afterward envisaged in pseudocapacitor applications. The MnO2, evidenced through Raman and X-ray photoelectron spectroscopy analysis, electrode composed of nanoplate-type surface morphology is hydrophilic and amorphous in nature. The electrochemical properties of MnO2 are examined using cyclic voltammetry, galvanostatic charge-discharge, and electrochemical impedance spectroscopy measurements in Na2SO4, NaOH, and KOH electrolytes, which demonstrate the pseudocapacitive signature with higher performance in Na2SO4 electrolyte than the others. A maximum specific capacitance of 804 F g−1 at a scan rate of 5 mV s−1 within −0.3–1.0 V potential range, with 84% retention after 1000 cycles, in 1 M Na2SO4 is evidenced.








Similar content being viewed by others
References
Alberto C, Ruiz C, Bélanger D, Rochefort D (2013) Electrochemical and spectro-electrochemical evidence of redox transitions involving protons in thin MnO2 electrodes in protic ionic liquids. Phys Chem C 117:20397–20405
Kotz R, Carlen M (2000) Principles and applications of electrochemical capacitors. Electrochim Acta 45:2483–2498
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Jayalakshmi M, Balasubramanian K (2008) Simple capacitors to supercapacitors—an overview. Electrochem Sci 3:1196–1217
Yu G, Hu L, Vosgueritchian M, Wang H, Xie X, Cui J, Cui Y, Bao Z (2011) Solution-Processe graphene/MnO2 nanostructured textiles for high performance electrochemical capacitors. Nano Lett 11:2905–2911
Conway BE (1999) Electrochemical supercapacitors. Scientific fundamentals and technological applications. Kluwer Academic, Plenum Publishers
Huang J, Sumpter B, Meunier V (2008) Theoretical model for nanoporous carbon supercapacitors. Chem Int Ed 47:520–524
Navale ST, Mali VV, Pawar SA, Mane RS, Naushad M, Stadler FJ, Patil VB (2015) Electrochemical supercapacitor development based on electrodeposited nickel oxide film. RSC Adv 5:51961–51965
Ghosh S, Inganas O (1999) Conducting polymer hydrogels as 3D electrodes applications for supercapacitors. Adv Mater 11:1214–1218
Toupin M, Brousse T, Belanger D (2002) Influence of microstructure on the charge storage properties of chemically synthesized manganese dioxide. Chem Mater 14:3946–3952
Reddy RN, Reddy RG (2003) Sol-gel MnO2 as an electrode material for electrochemical capacitors. Power Sources 124:330–337
Pang SC, Anderson MA, Chapman TW (2000) Novel electrode materials for thin-film ultracapacitor comparison of electrochemical properties of sol-gel-derived and electrodeposited manganese dioxide. Electrochem Soc 147:444–450
Chiung JK, Huang C, Tsai WT, Deng MJ, Sun I, Chen P (2008) Manganese films electrodeposited at different potentials and temperatures in ionic liquid and their application as electrode materials for supercapacitors. Electrochim Acta 53:4447–4453
Gupta M, Pinisetty D, Flake JC, Spivey JJ (2010) Pulse electrodeposition of CuZnO and Mn–Cu–ZnO nanowires. Electrochem Soc 157:D473–D478
Wu J, Johnson CD, Jiang Y, Gemmen RS, Liu X (2008) Pulse plating of Mn–Co alloys for SOFC interconnect applications. Electrochim Acta 54:793–800
Wang J, Xu Y, Wang J, Du X, Xiao F, Li J (2010) High charge/discharge rate polypyrrole films prepared by pulse current polymerization. Synth Met 160:1826–1831
Chandrasekar MS, Pushpavanam M (2008) Pulse and pulse reverse plating conceptual advantages and applications. Electrochim Acta 53:3313–3322
Xiao F, Xu Y (2012) Pulse electrodeposition of manganese oxide for high rate capability supercapacitors. Electrochem Sci 7:7440–7450
Hassan S, Suzuki M, El-Moneim AA (2012) Capacitive behavior of manganese dioxide/stainless steel electrodes at different deposition currents. Mater Sci 2(2):11–14
Jadhav P, Suryawanshi M, Dalavi D, Patil D, Jo E, Kolekar S, Wali A, Karanjkar M, Kim J, Patil P (2015) Design and electro-synthesis of 3-D nanofibers of MnO2 thin films and their application in high performance supercapacitor. Electrochim Acta 176:523–532
Lee M, Chang J, Hsieh Y, Tsai W, Lin C (2010) Manganese oxide thin films prepared by potentiodynamic electrodeposition and their supercapacitor performance. Solid State Electrochem 14:1697–1703
Ali G, Yusoff MM, Ng YH, Lim HN, Feng CK (2015) Potentiostatic and galvanostatic electrodeposition of manganese oxide for supercapacitor application: a comparison study. Curr Appl Phys 15:1143–1147
Wang HQ, Yang G, Li QY, Zhong XX, Wang FP, Li ZS, Li Y (2011) Porous nano-MnO2: large scale synthesis via a facile quick-redox procedure and application in a supercapacitor. New J Chem 35:469–475
Toupin M, Brousse T, Belanger D (2004) Charge storage mechanism of MnO2 electrode used in aqueous electrochemical capacitor. Chem Mater 16:3184–3190
Nakayama M, Konoshi S, Tagashira H, Ogura K (2005) Electrochemical synthesis of layered manganese oxides intercalated with tetra alkylammonium ions. Langmuir 21:354–359
Hsu YK, Chen YC, Lin YG, Chen LC, Chen KH (2012) Birnessite-type manganese oxides nanosheets with hole acceptor assisted photo electrochemical activity in response to visible light. Mater Chem 22:2733–2739
Yu D, Yao J, Qiu L, Wang Y, Zhang X, Feng Y, Wang H (2014) In situ growth of Co3O4 nanoparticles on α-MnO2 nanotubes: a new hybrid for high-performance supercapacitors. Mater Chem A 2:8465–8471
Juliena C, Massotb M (2002) Spectroscopic studies of the local structure in positive electrodes for lithium batteries. Phys Chem 4:4226–4235
Qiu Y, Xu P, Guo B, Cheng Z, Fan H, Yang M, Yang X, Li J (2014) Electrodeposition of manganese dioxide film on activated carbon paper and application for supercapacitor with high rate capability. RSC Adv 4:64187–64192
Hedborg E, Winquist F, Lundstrom I (1994) Influence of wettability on the properties of thin porous platinum films as gates of metal-oxide-semiconductor devices in electrolytes. Thin Solid Films 240:147–151
Gao H, Xiao F, Ching C, Duan H (2012) High performance asymmetric supercapacitor based on graphene hydrogel and nanostructured MnO2. ACS Appl Mater Interfaces 4:2801–2810
Yan D, Yan P, Cheng S, Chen J, Zhuo R, Feng J, Zhang G (2009) Fabrication In-depth characterization, and formation mechanism of crystalline porous Birnessite MnO2 film with amorphous bottom layers by hydrothermal method. Cryst Growth Des 9:218–222
Kadam S, Padwal P, Mane S, Kulkarni S (2016) Electrochemical synthesis and investigation of nano MnO2 electrode material for supercapacitor application. Adv Mater Proc 1(2):205–209
More PD, Jadhav PR, Ingole SM, Navale YH, Patil VB (2017) Preparation, structural and electrochemical supercapacitive properties of sprayed manganese oxide film electrode. J Mater Sci Mat in Electron 28(1):707–714
Gujar TP, Kim W, Puspitasari I, Jung KD, Joo OS (2007) Electrochemically deposited Nanograin ruthenium oxide as a pseudocapacitive electrode. Electrochem Sci 22:666–673
Xu C, Li B, Du H, Kang F, Zeng Y (2008) Electrochemical properties of nanosized hydrous manganese dioxide synthesized by a self-reacting microemulsion method. Power Sources 180:664–670
Yuan CZ, Gao B, Zhang XG (2007) Electrochemical capacitance of NiO/Ru0.35V0.65O2 asymmetric electrochemical capacitor. J Power Sources 173:606–612
Navale YH, Ingole SM, Navale ST, Stadler FJ, Mane RS, Naushad M, Patil VB (2017) Electro-synthesized fibrous polyaniline electrode as an active electrochemical supercapacitor material. J Colloid Interface Sci 487:458
Zhao J, Tang B, Cao J, Feng J, Liu P, Zhao J, Xu J (2012) Effect of hydrothermal temperature on the structure and electrochemical performance of manganese compound/ordered mesoporous carbon composites for supercapacitors. Mater Manuf Process 27(2):119–124
Taberna PL, Simon P, Fauvarque JF (2003) Electrochemical characteristics and impedance spectroscopy studies of carbon-carbon supercapacitors. Electrochem Soc 150:A292–A300
Ganesh V, Pitchumani S, Lakshminarayanan V (2006) New symmetric and asymmetric supercapacitors based on high surface area porous nickel and activated carbon. Power Sources 158:1523–1532
Wang BY, Guo PZ, Bi HQ, Li Q, Zhang GL, Wang RY, Liu JQ, Zhao XS (2013) Electrocapacitive properties of MnFe2O4 electrodes in aqueous LiNO3 electrolyte with surfactants. Electrochem Sci 8:8966–8977
Acknowledgements
Professor V.B. Patil would like thank CSIR, for financial support through the scheme no. 3 (1319)/14/EMR-II, RUSA Maharashtra (scheme no. RUSA/R&I/2016/267) and also to DAE-BRNS, India, for financial support through the scheme no. 34/14/21/2015-BRNS.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ingole, S.M., Navale, S.T., Navale, Y.H. et al. Galvanostatically electroplated MnO2 nanoplate-type electrode for potential electrochemical pseudocapacitor application. J Solid State Electrochem 21, 1817–1826 (2017). https://doi.org/10.1007/s10008-017-3557-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-017-3557-8